Article contents
Laboratory Soft X-Ray Microscopy with an Integrated Visible-Light Microscope—Correlative Workflow for Faster 3D Cell Imaging
Published online by Cambridge University Press: 07 October 2020
Abstract
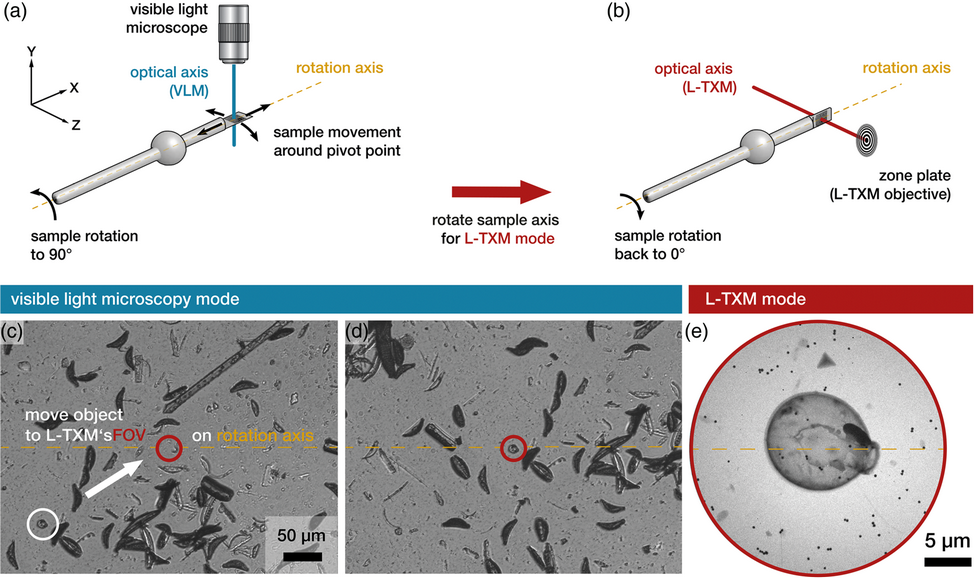
Laboratory transmission soft X-ray microscopy (L-TXM) has emerged as a complementary tool to synchrotron-based TXM and high-resolution biomedical 3D imaging in general in recent years. However, two major operational challenges in L-TXM still need to be addressed: a small field of view and a potentially misaligned rotation stage. As it is not possible to alter the magnification during operation, the field of view in L-TXM is usually limited to a few tens of micrometers. This complicates locating areas and objects of interest in the sample. Additionally, if the rotation axis of the sample stage cannot be adjusted prior to the experiments, an efficient workflow for tomographic imaging cannot be established, as refocusing and sample repositioning will become necessary after each recorded projection. Both these limitations have been overcome with the integration of a visible-light microscope (VLM) into the L-TXM system. Here, we describe the calibration procedure of the goniometer sample stage and the integrated VLM and present the resulting 3D imaging of a test sample. In addition, utilizing this newly integrated VLM, the extracellular matrix of cryofixed THP-1 cells (human acute monocytic leukemia cells) was visualized by L-TXM for the first time in the context of an ongoing biomedical research project.
Keywords
- Type
- Software and Instrumentation
- Information
- Copyright
- Copyright © Microscopy Society of America 2020
References
- 9
- Cited by


