Published online by Cambridge University Press: 07 January 2022
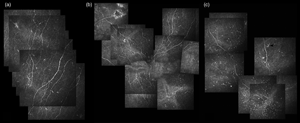
The present study investigated the corneal sub-basal nerve plexus (SNP) modifications in glaucoma. Ninety-five glaucomatous patients were enrolled and divided into Group 1 and 2, preserved and preservative-free mono-therapy (30 and 28 patients), and Group 3, multi-therapy (37). Thirty patients with dry eye disease (DED) and 32 healthy subjects (HC) served as controls. In vivo confocal microscopy evaluated the nerve fibers density (CNFD), length (CNFL), thickness (CNFT), branching density (CNBD), and dendritic cell density (DCD). CNFD, CNFL, and CNBD were reduced in Group 3 and DED compared to HC (p < 0.05). CNFL was reduced in Group 3 compared to Group 2 (p < 0.05), and in Group 1 compared to HC (p < 0.001). CNFD, CNBD, and CNFT did not differ between glaucomatous groups. DCD was higher in Group 3 and DED compared to HC and Group 2 (p < 0.01). Group 3 showed worse ocular surface disease index (OSDI) scores compared to Group 1, 2, and HC (p < 0.05). CNFL and DCD correlated with OSDI score in Group 3 (r = −0.658, p < 0.001; r = 0.699, p = 0.002). Medical therapy for glaucoma harms the corneal nerves, especially in multi-therapy regimens. Given the relations with the OSDI score, SNP changes seem features of glaucoma therapy-related OSD and negatively affects the patient's quality of life.
LA and LB equally contributed to this paper and share primary authorship.