Published online by Cambridge University Press: 10 June 2020
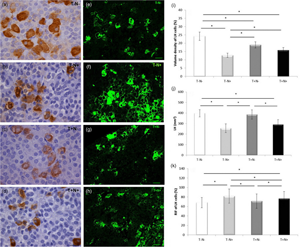
The aim of the study was to investigate the effects of chronic nandrolone decanoate treatment and/or swimming training on immunohistomorphometric parameters on rat pituitary gonadotropic cells. Male Wistar albino rats, 10 weeks old, were classified into four groups: control (T−N−), nandrolone (T−N+), swimming training (T+N−), and swimming training with nandrolone (T+N+). The T+ groups swam for 4 weeks, 1 h/day, 5 days/week. The N+ groups received nandrolone decanoate (20 mg/kg) once per week for 4 weeks. Pituitary tissue sections were processed and stained for immunohistochemical analysis and immunofluorescence. The volume density of luteinizing hormone (LH) cells was decreased by 48% in T−N+ and for 35% in the T+N+ group. The volume density of follicle-stimulating hormone (FSH) cells was decreased by 39% in T−N+ and for 30% in T+N+ compared to the control. Nandrolone alone, or combined with swimming training, decreased the number of LH/FSH cells compared to the control. The levels of the immunofluorescent signal of LH/FSH cells were increased in all experimental groups. Nandrolone alone decreased the serum level of LH by 17%, whereas swimming training alone increased FSH levels by 11% compared to the control. Serum levels of testosterone were increased in all experimental groups. Nandrolone alone, or combined with swimming training, decreased immunohistomorphometric parameters of gonadotropic cells, whereas the levels of immunofluorescent signal were increased.