Article contents
Histological and Ultrastructural Studies of the Unique Hemopoietic-Endocrine Organ of the Grass Carp, Ctenopharyngodon idella (Valenciennes, 1844)
Published online by Cambridge University Press: 13 October 2020
Abstract
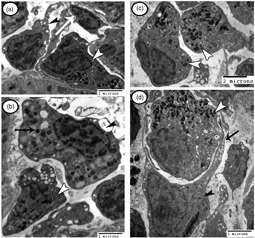
Specific features of the immunohistochemical and ultrastructural organization of the hemopoietic head-kidney (HK) in adult Ctenopharyngodon idella (Valenciennes, 1844) were investigated using light and transmission electron microscopy. The HK of grass carp possessed all developmental stages of leucocytes and erythrocytes, as well as dendritic cells and epithelial reticular cells. The rodlet cells were expressed α-smooth muscle actin (SMA). In addition, macrophages were the most numerous cells in the HK, which aggregated into structures called melanomacrophage centers (MMCs). On contrary, the chromaffin and interrenal cells (ICs) were mixed and organized into large anastomosing cords, which lined the posterior cardinal veins of the HK, and associated with many blood capillaries. The ICs displayed the characteristic features of steroid-producing cells. Three types of chromaffin cells: adrenaline, noradrenaline, and small granule-containing cells were observed in the HK. Glial fibrillary acidic protein (GFAP)-positive sustentacular cells were marked among the chromaffin cells. Hemopoietic cells, immune cells, MMCs, rodlet cells, in addition to three types of chromaffin cells and one type of interrenal cells in the HK were correlated with the functional significance of the fish concerned.
- Type
- Micrographia
- Information
- Copyright
- Copyright © The Author(s), 2020. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 3
- Cited by


