Published online by Cambridge University Press: 09 March 2022
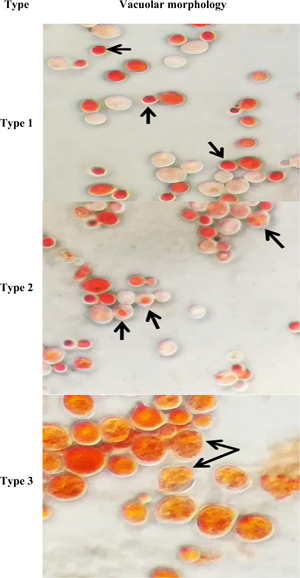
Polyalthia longifolia is known for its anti-oxidative properties, which might contribute to the antiaging action. Hence, the current research was conducted to evaluate the antiaging activity of P. longifolia leaf methanolic extract (PLME) in a yeast model based on morphology using microscopic approaches. Saccharomyces cerevisiae BY611 strain yeast cells were treated with 1.00 mg/mL of PLME. The antiaging activity was assessed by determining the replicative lifespan, total lifespan, vacuole morphology by light microscopy, extra-morphology by scanning (SEM), and intra-morphology by transmission (TEM) electron microscopy. The findings demonstrated that PLME treatment significantly accelerated the replicative and total lifespan of the yeast cells. PLME treatment also delays the formation of large apoptotic-like type 3 yeast cell vacuoles. The untreated yeast cells demonstrated aging morphology via SEM analysis, such as shrinking, regional invaginations, and wrinkled cell surface. The TEM analysis revealed the quintessential aging intracellular morphology such as swollen, wrinkled, or damaged vacuole formation of the circular endoplasmic reticulum, a rupture in the nuclear membrane, fragmentation of the nucleus, and complete damaged cytoplasm. Decisively, the present study revealed the vital role of PLME in the induction of antiaging activity in a yeast model using three microscopic approaches—SEM, TEM, and bright-field light microscope.