Published online by Cambridge University Press: 07 December 2020
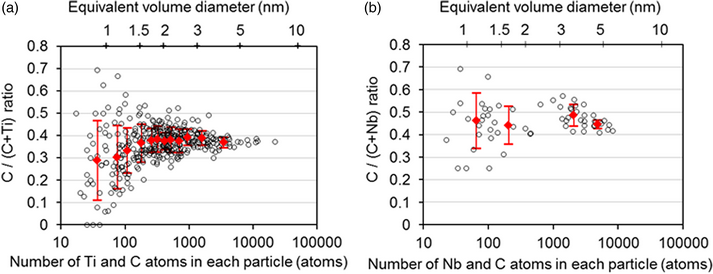
The carbon (C) ratios, namely the atomic ratios of C/(C + M), in nano-sized coherent MC precipitates (M = Ti, Nb) with the NaCl-type (B1) structure in ferritic steels, which had been isothermally aged at 580 °C, were investigated using atom probe tomography (APT). Considering the influences of the trajectory aberration, detection loss, and peak overlap, we determined the C ratios to be ~0.40 and ~0.45 for an equivalent volume diameter of 1.5–5 nm and 1–5 nm for the TiC and NbC precipitates, respectively, suggesting that there is a considerable fraction of C vacancies in both nano-sized precipitates. The apparent C ratios show significant scatter with decreasing particle size, while the apparent mean C ratios of very fine TiC particles, smaller than 1.5 nm, decreased with decreasing particle size. With the use of one of the latest APT instruments with a high detection efficiency, the scattering in the apparent C ratios was reduced because the counting statistics were improved; however, the artificial enrichment of C atoms to particular crystallographic directions of ferrite hindered the determination of the C ratio for very fine TiC particles smaller than 1.5 nm.