Published online by Cambridge University Press: 24 November 2021
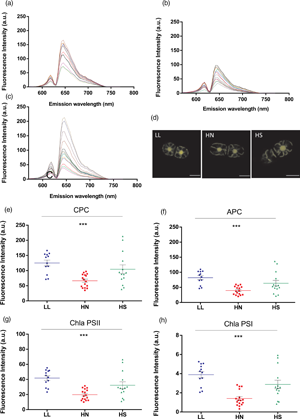
Alga in the genus Chroothece have been reported mostly from aquatic or subaerial continental environments, where they grow in extreme conditions. The strain Chroothece mobilis MAESE 20.29 was exposed to different light intensities, red and green monochromatic light, ultraviolet (UV) radiation, high nitrogen concentrations, and high salinity to assess the effect of those environmental parameters on its growth. Confocal laser scanning microscopy (CLSM) was used as an “in vivo” noninvasive single-cell method for the study. The strain seemed to prefer fairly high light intensities and showed a significant increase in allophycocyanin (APC) and chlorophyll a [photosystem I (PSI) and photosystem II (PSII)] fluorescence with 330 and 789 μM/cm2/s intensities. Green monochromatic light promoted a significant increase in the fluorescence of APC and chlorophyll a (PSI and PSII). UV-A significantly decreased phycocyanin and increased APC, while UV-A + B showed a greater decreasing effect on c-Phycocyanin but did not significantly change concentrations of APC. The increase in nitrogen concentration in the culture medium significantly and negatively affected all pigments, and no effect was observed with an increase in salinity. Our data show that CLSM represents a very powerful tool for ecological research of microalgae in small volumes and may contribute to the knowledge of phycobiliproteins in vivo behavior and the parameters for the large-scale production of these pigments.