No CrossRef data available.
Article contents
Calcium, Ca2+-ATPase, Calmodulin, and Calbindin D-28KD Localization in Testis of Leptodactylus chaquensis (Anura: Leptodactylidae)
Published online by Cambridge University Press: 17 March 2022
Abstract
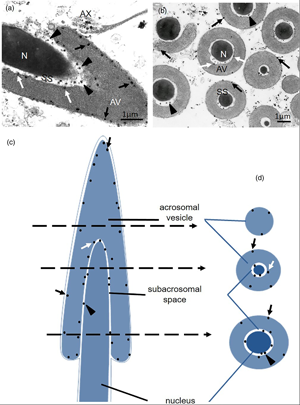
The intracellular localization of Ca2+, Ca2+-ATPase, Calmodulin, and Calbindin D-28KD have been studied in testes of the toad Leptodactylus chaquensis, using ultracytochemical and immunohistochemical techniques. The Ca2+ presences in the nucleus and into the mitochondria of the germ cells, together with the activity of Ca2+-ATPase detected in the nuclear envelope and mitochondrial crests, suggest the participation of this transporter in the storage of Ca2+. In Sertoli cells, Ca2+ deposits were also found in vesicles and lamellar bodies. Calmodulin and Calbindin D-28KD were revealed in the cytoplasm of both cell types. At the spermatozoon level, the cation deposits were located in the subacrosomal space and in the acrosomal vesicle. Ca2+-ATPase activity was observed in the acrosomal and plasma membranes of the gamete that suggests the existence of a transport system responsible for maintaining low cytoplasmic Ca2+ levels. The activity of Ca2+-ATPase and the location of Ca2+ deposits in gamete tail would be related to flagellar movement. The colocalization of Ca2+ deposits and their binding proteins in efferent duct cells would probably be associated with secretory activity. Considering that intracellular Ca2+ is present in different gonadal cells, this work would provide a better understanding of the cation importance in the testicular functions of this species.
- Type
- Micrographia
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of the Microscopy Society of America


