Introduction
The halogens, fluorine (F), chlorine (Cl), bromine (Br) and iodine (I), exert an important role in many magmatic and hydrothermal processes (Harlov & Aranovich, Reference Harlov and Aranovich2018). Nevertheless, their geochemical behavior in most geological settings is not well understood, mostly due to a lack of reliable geochemical analyses (see Klemme & Stalder (Reference Klemme, Stalder, Harlov and Aranovich2018) for some discussion). Halogens can be analyzed in geological samples using various analytical techniques, including secondary ion mass spectrometry (SIMS) (e.g., Hinton, Reference Hinton1990; Straub & Layne, Reference Straub and Layne2003; Kusebauch et al., Reference Kusebauch, John, Whitehouse, Klemme and Putnis2015; Cadoux et al., Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017), noble gas mass spectrometry (e.g., Kendrick et al., Reference Kendrick, Kamenetsky, Phillips and Honda2012), instrumental neutron activation (INAA) (e.g., Marks et al., Reference Marks, Wenzel, Whitehouse, Loose, Zack, Barth, Worgard, Krasz, Eby, Stosnach and Markl2012; Cadoux et al., Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017), microbeam X-ray fluorescence (μ-XRF) spectrometry (e.g., Pan & Dong, Reference Pan and Dong2003), laser ablation inductively coupled mass spectrometry (LA-ICP-MS) (e.g., Hammerli et al., Reference Hammerli, Rusk, Spandler, Emsbo and Oliver2013) and electron probe microanalysis (EPMA) (e.g., Zhang et al., Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016, Reference Zhang, Lin, Pan, Feng, Almeev and Holtz2017). Compared with most of the methods mentioned above, advantages of EPMA are (a) the availability in most geoscience institutes, (b) excellent spatial resolution, (c) relatively low analytical costs, and (d) simultaneous determination of all four halogens. Until recently though, halogen analyses with EPMA were hindered by relatively high detection limits (DLs), e.g., 600–1,200 μg/g for F, due to low count rates when using thallium acid phthalate (TAP) crystals (Potts & Tindle, Reference Potts and Tindle1989), or spectral interferences when using multilayered crystals (Potts & Tindle, Reference Potts and Tindle1989).
The main problems associated with EPMA of halogens are: (1) spectral interferences between Fe on F, Al on Br, and Ca on I in rock-forming minerals and glasses and (2) lack of well-characterized EPMA reference materials that cover the broad range of halogen concentrations in natural minerals and glasses as well as (3) lack of low Al and low Ca EPMA calibration materials to estimate correction factors for Br and I analyses.
As to spectral interferences, EPMA of reference materials like the Kakanui Hornblende (USNM 143965) (Jarosewich et al., Reference Jarosewich, Nelen and Norberg1980) and the Durango fluorapatite (Marks et al., Reference Marks, Wenzel, Whitehouse, Loose, Zack, Barth, Worgard, Krasz, Eby, Stosnach and Markl2012) revealed that the first-order FeLα and FeLβ, and the second-order MgKα and MgKβ spectral lines interfere with the FKα line. Marks et al. (Reference Marks, Wenzel, Whitehouse, Loose, Zack, Barth, Worgard, Krasz, Eby, Stosnach and Markl2012) showed that the FeLα line overlaps at the peak position of the FKα, while the MgKβ line overlaps at the right-side background position of the FKα. Bromine analyses are affected by interferences from Al-lines, e.g., as AlKα and AlKβ interfere with the adjacent BrLα and BrLβ lines (Fleet, Reference Fleet1989; Zhang et al., Reference Zhang, Lin, Pan, Feng, Almeev and Holtz2017). Finally, spectral interferences for ILα result from the Kβ line of Ca.
Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016, Reference Zhang, Lin, Pan, Feng, Almeev and Holtz2017) developed specific analytical protocols for EPMA analysis of F and Br in geological samples. Analysis of F with a TAP crystal is possible as there is no spectral interference, but obtained count rates are usually too low for meaningful analysis (Zhang et al., Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016), unless more than one TAP crystals are used (Bénard et al., 2017). Instead, F is preferentially measured using a W-Si-multilayered pseudocrystal as the diffraction crystal (Zhang et al., Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016). This is the method of choice, as higher count rates result in lower DLs (82–106 μg/g for F). As to the overlap on FKα from the second-order MgKβ line, Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016) used the EPMA in a differential mode with an optimized PHA (pulse height analysis) setting in signal processing, which completely eliminated interferences from the second-order MgKβ line. The overlapping first-order FeLα on FKα peak cannot be filtered by modifying the PHA settings and was instead calibrated quantitatively using a few F-free, Fe-bearing natural silicate glasses and minerals. Using this approach, Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016) demonstrate the successful analysis of F in a number of reference glasses (VG-2, VG-A99, VG-568, and BCR-2G), as well as in synthetic glasses made from powders of some rock reference materials (AC-E, GS-N, and DR-N).
For the EPMA analysis of Br, Zhang et al. (Reference Zhang, Lin, Pan, Feng, Almeev and Holtz2017) established an analytical protocol by using Br-free but Al-bearing materials to quantify the overlap from AlKα on BrLβ lines. The count rate of the BrLβ peak signal was enhanced by high beam currents (100–200 nA) and long measurement times (120 s at the peak position and 60 s at the background). The application of this protocol to Al- and Br-bearing materials, such as sodalite and scapolite, and to five synthetic glasses yielded Br concentrations in the range of 250–4,000 μg/g that were consistent with those measured by microbeam synchrotron X-ray fluorescence (μ-SXRF) spectrometry (Zhang et al., Reference Zhang, Lin, Pan, Feng, Almeev and Holtz2017). The EPMA method presented by Zhang et al. (Reference Zhang, Lin, Pan, Feng, Almeev and Holtz2017) has a Br detection limit of ~100–300 μg/g.
To our knowledge, no such EPMA protocol has been established for I analyses in natural and experimental silicate glasses. Iodine analyses are more complicated, as I concentrations in natural glasses are extremely low (i.e., tens to hundreds ng/g, Kendrick, Reference Kendrick2012; Kendrick et al., Reference Kendrick, Honda, Pettke, Scambelluri, Phillips and Giuliani2013; Reference Kendrick, Jackson, Hauri and Phillips2015), and EMPA detection limits (~150 μg/g; e.g., Lerouge et al., Reference Lerouge, Claret, Denecke, Wille, Falkenberg, Ramboz, Beny, Giffaut, Schäfer, Gaucher and Tournassat2010) are higher than those for F and Br. The EPMA analysis of Cl in geological samples requires no special treatment.
The second problem with halogen analysis of geological samples with the EPMA is due to the fact that well-characterized halogen-bearing reference materials are scarce. Recently, Cadoux et al. (Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017) produced Br-bearing silicate glasses with concentrations from 0.5 to 6,000 μg/g. Attempts to calibrate EPMA standards for Br in basaltic (with ~6,000 ppm Br; Cadoux et al., Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017) and haplogranite glass (with 0.96 ± 0.04 wt% Br, Louvel et al., Reference Louvel, Sanchez-Valle, Malfait, Pokrovski, Borca and Grolimund2020) have also been recently reported. Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016) synthesized F-bearing glasses in granitic and dioritic rock compositions, which have been used as reference materials for bulk halogen analyses. However, to our knowledge, no I-bearing reference material glasses are available.
In principle, halogens can be determined using various analytical techniques, e.g., SIMS (e.g., Straub & Layne, Reference Straub and Layne2003; Kusebauch et al., Reference Kusebauch, John, Whitehouse, Klemme and Putnis2015; Cadoux et al., Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017), noble gas method (e.g., Kendrick et al., Reference Kendrick, Kamenetsky, Phillips and Honda2012), INAA (e.g., Marks et al., Reference Marks, Wenzel, Whitehouse, Loose, Zack, Barth, Worgard, Krasz, Eby, Stosnach and Markl2012; Cadoux et al., Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017), μ-XRF spectrometry (e.g., Pang & Dong, Reference Pan and Dong2003), EPMA (e.g., Zhang et al., Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016, Reference Zhang, Lin, Pan, Feng, Almeev and Holtz2017), and others. Compared with most of the methods mentioned above, EPMA advantages are (a) the ease of access in such instruments, (b) reasonable spatial resolution (< 1 μm), (c) relatively low analytical costs, and (d) simultaneous determination of all four halogens. Until recently though, halogen analyses with EPMA were hindered by relatively high DLs (600–1,200 μg/g for F, while Br data are rare) and low count rates when using TAP crystals (Potts & Tindle, Reference Potts and Tindle1989), and spectral interferences when using multilayered crystals (Potts & Tindle, Reference Potts and Tindle1989). The analytical protocols by Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016, Reference Zhang, Lin, Pan, Feng, Almeev and Holtz2017) tackled the problem generated by spectral interferences in F and Br analyses, hence leading to higher count rates and lower DLs (106 μg/g for F and between 120 and 300 μg/g for Br). Still, these DLs, especially for Br and I, are too high for obtaining reasonable analyses in natural samples (although, in some case, Br concentrations can reach up to 300 μg/g in melt inclusions and matrix glasses; Bureau & Métrich, Reference Bureau and Métrich2003). But they can be particularly useful in experimental petrology in order to determine partition coefficients for these elements, especially I, in magmatic and/or hydrothermal systems, thus evaluating the behavior of these elements in such systems. However, further improvement of these analytical protocols is required, particularly an assessment of the methods at lower F and Br contents, improved DLs, and establishment of an analytical protocol for I analyses in geological materials.
Rationale
To improve the analytical EPMA techniques for the analysis of halogens in natural samples, we set out to prepare an improved analytical protocol. To achieve this, we prepared new synthetic halogen-free glasses in different compositions to improve the calibration of interferences during halogen analysis with EPMA, and we also present a new analytical protocol for I analyses in silicate glasses. Furthermore, we synthesized two halogen-bearing silicate glasses to examine the accuracy of our calibration for F, Br, and I. Fluorine and Br analyses of silicate glasses were conducted using the analytical protocols of Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016, Reference Zhang, Lin, Pan, Feng, Almeev and Holtz2017), and I using our own new analytical protocol. These new reference materials and improved EPMA methods can be applied to geological investigations that require high spatial resolution, e.g., experimental studies (e.g., Steenstra et al., Reference Steenstra, van Haaster, van Mulligen, Flemetakis, Berndt, Klemme and van Westrenen2020), or the study of melt inclusions (e.g., Métrich et al., Reference Métrich, Zanon, Créon, Hildenbrand, Moreira and Marques2014; Rose-Koga et al., Reference Rose-Koga, Koga, Moreira, Vlastelic, Jackson, Whitehouse, Shimizu and Habib2017; present study).
Materials and Methods
The EPMA methods of Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016, Reference Zhang, Lin, Pan, Feng, Almeev and Holtz2017) rely on a set of halogen-free reference materials to calibrate spectral interferences during EPMA measurements. In our laboratory, we use similar glasses (see below) but we also synthesized several new halogen-free glasses (Table 1) to improve the calibration of interferences during halogen analysis, and we also analyzed various halogen-bearing glasses, including well-known reference materials and newly synthesized glasses (Tables 2–5). All synthesis experiments were performed in the experimental petrology laboratory of the Institute for Mineralogy at the University of Münster, Germany. The halogen-free glasses are of basaltic, rhyolitic, and enstatitic composition and were synthesized using analytical grade oxides and salts in a piston-cylinder apparatus at high pressures and high temperatures (P = 1 GPa, T = 1,450°C, 36 h run duration), or in gas-mixing furnaces at atmospheric pressure (Table 1). Samples were loaded into Pt-capsules. The halogen-bearing glasses include all halogens (F, Cl, Br, and I) and their halogen concentrations are 1,000 and 5,000 μg/g for each (Table 5). Backscattered electron images of the resulting glasses showed that the samples were completely quenched, and EPMA analyses showed only small compositional heterogeneity (small errors, Tables 2–4).
Table 1. Major Elements (wt%) Composition and “Apparent” Halogen Contents (μg/g) of Halogen-Free Glasses Used for the Calibration.

Synthetic halogen-free glasses are used to establish the calibration lines. “F”, “Br,” and “I” refer to “apparent” halogen contents that are caused by the interferences of Fe on F-line, Al on Br-line, and Ca on I-line. The glasses were prepared by: 1Morlok et al. (Reference Morlok, Klemme, Weber, Stojic, Sohn and Hiesinger2017), 2Morlok et al. (Reference Morlok, Klemme, Weber, Stojic, Sohn, Hiesinger and Helbert2019), 3Berndt et al. (Reference Berndt, Koepke and Holtz2005), and 4present study. Refer to these studies for detailed information.
Table 2. Reference Glasses Analyzed in the Present Study and Their F, Cl contents (μg/g).

Glasses were analyzed with EPMA, and F and Cl concentrations were determined using our calibration lines (Figs. 1a–1c). Errors reported are 2σ – SE. The glasses were synthesized by 1Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016), 2Kendrick et al. (Reference Kendrick, Kamenetsky, Phillips and Honda2012), and 3Cadoux et al. (Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017). Cl results are from Cadoux (personal communication).
Table 3. Fluorine and Cl Contents of Reference Glasses from the Literature Compared With Our Study.

1Thordarson et al. (Reference Thordarson, Self, Óskarsson and Hulsebosch1996); 2Kamenetsky et al. (Reference Kamenetsky, Everard, Crawford, Varne, Eggins and Lanyon2000); 3Kamenetsky & Maas (Reference Kamenetsky and Maas2002); 4Straub & Layne (Reference Straub and Layne2003); 5Michel & Villemant (Reference Michel and Villemant2003); 6Witter & Kuehner (Reference Witter and Kuehner2004); 7Streck & Wacaster (Reference Streck and Wacaster2006); 8van der Zwan et al. (Reference van der Zwan, Fietzke and Devey2012); 9Kendrick et al. (Reference Kendrick, Kamenetsky, Phillips and Honda2012); 10Wang et al. (Reference Wang, Marks, Keller and Markl2014); 11Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016); 12Marks et al. (Reference Marks, Kendrick, Eby, Zack and Wenzel2016), Cadoux (personal communication). Errors reported are either SD, 1σ or 2σ depending on the study. The reported accuracy of our measurements is relative to the different methods (Column “Analytical Method") used in different studies.
Table 4. Bromine Contents (μg/g) and Accuracy of Reference Glasses Analyzed in Our Study and in Cadoux et al. (Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017).

Bromine concentrations were obtained using our calibration line. Errors reported from Cadoux et al. (Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017) and the present study are 2σ – SE. 1Cadoux et al. (Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017) and 2present study. The reported accuracy of our measurements is relative to the different analytical methods (Column “Analytical Method") used in different studies.
Table 5. Major Elements (wt%) and Halogen Concentrations (μg/g) of Experimental Glasses.

Halogen-bearing synthetic experimental glasses are SF-G8 and -G9 (this study). The nominal starting material compositions for each halogen were 5,000 μg/g for SF-G8 and 1,000 μg/g for SF-G9.
The new halogen-free glasses were used to quantify the Fe, Al, and Ca interferences on F, Br, and I peaks, and this allowed us to construct calibration lines (Figs. 1a–1c) that can be used to subtract the amount of “apparent” halogen (Table 1), arising from the interfering Fe, Mg, Al, or Ca. To evaluate the accuracy of our method, we analyzed several independent reference material glasses available in the literature (Tables 2–4).

Fig. 1. (a) Calibration line for F. Glasses with different Fe contents were used from ~0.4 to ~14 wt% (Table 1). There is an almost linear correlation (R 2 = 0.9962) with increasing Fe content. The 95.45% confidence line indicates that the error (2 SD) on the F content because of Fe is relatively small (±40 μg/g) up to ~9 wt% and increases (±80 μg/g) at higher concentrations. (b) Calibration line for Br that quantifies the effect of Al in the analysis signal. Our glasses have an Al2O3 content ranging from ~1 to ~18 wt% (Table 1). The excess Br signal we get from Al also shows a linear relation (R 2 = 0.9891) with an increasing amount of Al inside the samples. Based on the 95.45% confidence lines, the error (2 SD) at the excess Br for Al concentrations higher than 12 wt% is ±20 μg/g, while it increases significantly at concentrations less than 2 wt%, probably due to the absence of glasses with very low Al contents. (c) Calibration line for I shows the effect of Ca-interference on the I measurements. The Ca compositional range of our glasses is from 0.4 to ~14 wt% (Table 1). The correlation between the glasses with different Ca contents and the excess I in the signal of the analysis has a linear correlation (R 2 = 0.9958). The 95% confidence lines show that the error (2 SD) at the excess I for Ca concentrations from ~5 to ~14 wt% is ±20 μg/g, while it increases at low concentrations (<2 wt%) at 40 μg/g.
Analytical Methods
All samples were examined with a JEOL 6510LV scanning electron microscope (SEM), and the major element concentrations of all phases were determined with a 5-spectrometer JEOL JXA 8530F electron microprobe analyzer (EPMA) at the Institute für Mineralogie at the Westfälische Wilhelms-Universität Münster (WWU). Halogen-bearing silicate glasses were measured in a first step for concentrations of Na, Mg, Al, Si, K, Ca, Ti, Cr, Mn, and Fe. Conditions were 15 kV accelerating voltage, 5–15 nA beam current, and counting times of 10 s on the peak and 5 s on the background positions, except for Na and K (7 s on peak and 3 s on background). Spot size was varied of between 10 and 20 μm. In a second step, halogens of the previously analyzed spots were measured with a beam current of 60 nA and counting times of 120 s on the peak and 60 s on the background (Zhang et al., Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016). The individual sets of silicate and halogen analyses were eventually merged using the JEOL WDS software package to obtain full φ(ρZ) matrix-corrected (Armstrong, Reference Armstrong1984) quantitative analysis. Spectral overlaps of FeLα on FKα, AlKα on BrLβ, and CaKβ on ILα were finally corrected using the previously determined correction factors. Details of EPMA halogen settings are summarized in Supplementary Table 1. Astimex Tugtupite, fluorite, and topaz were used as reference materials for F and Cl analyses, and Astimex TlBrI was used as a reference material for Br and I. Detection limits (DLs) are ~88 μg/g for F, ~28 μg/g for Cl, ~156 μg/g for Br, and ~76 μg/g for I, and were determined by the JEOL software using background statistics and the 3σ criterion from equation 9.25 as described in Goldstein et al. (Reference Goldstein, Newbury, Michael, Ritchie, Scott and Joy2018).
Results and Discussion
Calibration Lines
Electron microprobe analysis of F, Br, and I of geological samples is difficult due to interferences of Fe on F-lines, Al on Br-lines, and Ca on I-lines. Hence, we used Fe-, Al-, and Ca-bearing glasses to calibrate the aforementioned interferences. Here, it should be noted that the calibration lines (i.e., the measured Fe–F, Al–Br and Ca–Cl contents of the silicate glasses) can vary with beam current and other settings (e.g., baseline, window, bias, and gain). This indicates that the calibration lines (and equations) reported further below and in Figures 1a–1c will not be the same for other laboratories with a different instrument type and/or a different measurement setting. Thus, for other instrument types and/or settings, new calibrations (following the same procedure) need to be performed, which are specifically designed for them. Moreover, to further assess the accuracy of the measurements, the calibrations for the measured elements should be periodically performed during each measurement session.
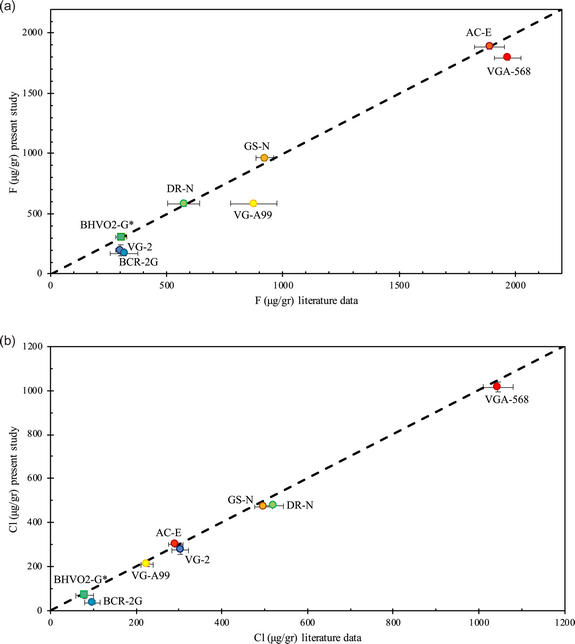
The calibration curves are depicted in Figures 1a–1c, and results are given in Tables 2 and 3. As an example, our analyses show a linear relationship between the “apparent” F signal and the Fe concentrations of the samples (Zhang et al., Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016), and the results may be described by the following linear equation: y = 65.209x + 287.93 with R 2 = 0.9962. The 2σ uncertainty on the “apparent” F content is ±40 μg/g but increases to ±80 μg/g for elevated Fe contents (>9 wt%). We then use this calibration line (Fig. 1a) to correct our measured F concentrations in various synthetic and natural halogen-bearing glasses from the literature, which have been used to check for the accuracy of halogen analyses in previous studies (Kamenetsky et al., Reference Kamenetsky, Everard, Crawford, Varne, Eggins and Lanyon2000; Kamenetsky & Maas, Reference Kamenetsky and Maas2002; Michel & Villemant, Reference Michel and Villemant2003; Straub & Layne, Reference Straub and Layne2003; Kendrick et al., Reference Kendrick, Kamenetsky, Phillips and Honda2012; Marks et al., Reference Marks, Wenzel, Whitehouse, Loose, Zack, Barth, Worgard, Krasz, Eby, Stosnach and Markl2012, Reference Marks, Kendrick, Eby, Zack and Wenzel2016; Wang et al., Reference Wang, Marks, Keller and Markl2014; Zhang et al., Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016). Details of the glasses we analyzed [VG-2 (USNM 111240/52, basaltic glass), VG-A99 (USNM 113498/1, basaltic glass), VG-568 (USNM 7285, rhyolitic glass), NMNH 113716/11 (USNM, basaltic glass), BCR-2G (USGS, basaltic glass), GSE1-G (USGS, basaltic glass), and BHVO2-G (USGS, basaltic glass)], the synthesized glasses from Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016), AC-E (granite), GS-N (granite) and DR-N (diorite), and glass 47963 (basaltic glass) (Kamenetsky et al., Reference Kamenetsky, Everard, Crawford, Varne, Eggins and Lanyon2000; Kamenetsky & Maas, Reference Kamenetsky and Maas2002, in Kendrick et al., Reference Kendrick, Kamenetsky, Phillips and Honda2012) are presented in Tables 2 and 3. We then compared our results with literature values by Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016), because we applied the same analytical rationale and one value from Marks et al. (Reference Marks, Kendrick, Eby, Zack and Wenzel2016) (Fig. 2a). The F precisions and accuracies of our analyses are given in Tables 2 and 3.

Fig. 2. (a) Fluorine literature data for the glasses VG-A99, VGA-568, VG-2, AC-E, DR-N, GS-N, BCR-2G, and BHVO2-G (Marks et al., Reference Marks, Kendrick, Eby, Zack and Wenzel2016; Zhang et al., Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016), compared with F data from the present study. Fluorine concentrations from each study can be found in Tables 2 and 3. The dashed line represents the 1:1 line. Our results are plotted mainly against the data from Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016), in order to show the good agreement of the individual studies. Glasses VGA-568, VG-A99, and VG-2 show differences that are discussed inside the text and are attributed to glass inhomogeneity. (b) Chlorine literature data for the glasses VG-A99, VGA-568, VG-2, AC-E, DR-N, GS-N, BCR-2G, and BHVO2-G (Marks et al., Reference Marks, Kendrick, Eby, Zack and Wenzel2016; Zhang et al., Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016), compared with Cl data from the present study. Chlorine concentrations from each study can be found in Tables 2 and 3. The dashed line represents the 1:1 line. Our results are plotted mainly against the data from Zhang et al. (Reference Marks, Kendrick, Eby, Zack and Wenzel2016) and show an overall excellent agreement.
To account for the interference of Al- on Br-lines during EPMA analysis, we followed an identical approach (Fig. 1b). The “apparent” Br signal and the Al concentrations of the samples also follow a linear relationship, with y = 48.467x + 7.7809 with an R 2 = 0.9891. At Al2O3 contents <12 wt%, 2σ uncertainty of the “apparent” Br of the sample increases from ~40 to ~80 μg/g, mainly due to the lack of Br-free reference glasses with such low concentrations of Al2O3. We then used the new calibration line to analyze Br-bearing hydrous glasses from Cadoux et al. (Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017), which were synthesized from natural volcanic rocks in various compositions (Table 2 and 3) and analyzed with in situ techniques such as SIMS, LA-ICP-MS, and synchrotron radiation X‐ray fluorescence spectrometry (SR-XRF) (Cadoux et al., Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017). Our EPMA results agree well with the data from Cadoux et al. (Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017) (Table 4).
We also prepared Ca-bearing I-free glasses which allowed us to quantify the effect of Ca in the signal of the I spectrum (~20–40 μg/g, Fig. 1c) during EPM analysis. Similar to F and Br, the “apparent” I signal shows a linear relationship with the Ca concentration of the samples, with y = 43.179x + 37.555 with an R 2 = 0.9958. As there are no available reference glasses for I analyses, we could not apply our new method to the aforementioned glasses.
The analysis of Cl with EPMA, however, does not require correction. We, therefore, measured the Cl content of the same synthetic and natural halogen-bearing glasses (Table 2) and then compared our results with the literature (Kamenetsky et al., Reference Kamenetsky, Everard, Crawford, Varne, Eggins and Lanyon2000; Kamenetsky & Maas, Reference Kamenetsky and Maas2002; Michel & Villemant, Reference Michel and Villemant2003; Straub & Layne, Reference Straub and Layne2003; Kendrick et al., Reference Kendrick, Kamenetsky, Phillips and Honda2012; Marks et al., Reference Marks, Wenzel, Whitehouse, Loose, Zack, Barth, Worgard, Krasz, Eby, Stosnach and Markl2012, Reference Marks, Kendrick, Eby, Zack and Wenzel2016; Wang et al., Reference Wang, Marks, Keller and Markl2014; Zhang et al., Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016). Chlorine contents of analyzed glasses along with the accuracy (relative to other studies) and precision of our measurements are summarized in Tables 2 and 3. We also compared our data with the results from Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016), and one value from Marks et al. (Reference Marks, Kendrick, Eby, Zack and Wenzel2016) (Fig. 2b).
Fluorine
Fluorine precision of the analyzed glasses (Table 2) ranges between ~1 and 27%. Glasses GSE1-G (228 ± 24 μg/g), BCR2-G (167 ± 19 μg/g), VG-2 (197 ± 46 μg/g), and NMNH 113716/11 (153 ± 41 μg/g) show a precision that varies from 10.5 to 27%. For the rest of the glasses, i.e., VG-568 (1,794 ± 21 μg/g), 47963 (989 ± 15 μg/g), VGA-99 (585 ± 26 μg/g), AC-E (1,885 ± 20 μg/g), GS-N (963 ± 22 μg/g), DR-N (586 ± 20 μg/g), BHVO2-G (305 ± 15 μg/g), precision ranges from 1 to 4.9% (Table 2).
The accuracy of the F analyses between individual studies and the present study varies from <1 to up to 63% and seems independent of absolute F contents. Our results for the glasses, VG-568, AC-E, GS-N, DR-N, BVO2-G, and 47963, agree with published literature values with an accuracy ranging from <0.2 to 9% (Table 3), especially with the results from Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016) (Fig. 2a). Reference glasses VG-A99, VG-2, BCR2-G, and GSE1-G show the lowest accuracy between the individual studies (Table 3). In VG-A99, our results, compared with the studies of Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016), Witter & Kuehner (Reference Witter and Kuehner2004), and Straub & Layne (Reference Straub and Layne2003), show an accuracy that ranges between 17 and 40%. For BCR-2G, compared with Michel & Villemant (Reference Michel and Villemant2003) and Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016), the accuracy is 63 and 47% respectively. For VG-2, the accuracy is 44 and 49%, compared with Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016) and Straub & Layne (Reference Straub and Layne2003), respectively. Marks et al. (Reference Marks, Kendrick, Eby, Zack and Wenzel2016) is the only study that reported values for GSE1-G, and in this case, the accuracy is 52%. All the F contents, errors, accuracy, and analytical methods used, for each study, can be found in Table 3.
In summary, we find overall good agreement of our EPMA data with analytical results of previous studies, and this provides a strong support for the validity of our new analytical method. Note that our EPMA data for some glasses differ from the literature results in terms of F (Table 3, glasses VG-A99, VG2, BCR2-G, and GSE-1G). The reasons for this are unclear, but this may be due to heterogeneity of F in the samples. Therefore, we conclude that glasses VG-568, AC-E, GS-N, DR-N, BVO2-G, and 47963 are suitable as reference materials for EPMA analysis, while we would caution the reader to use VG-A99, VG-2, BCR-2G, and GSE-1G as reference materials for the micro-analysis of F. Here, it must be noted that accuracy is how well the values match with the “true” concentration of an element, in this case F. But the literature values are far from being true as there are either not enough data available to give a “compiled” true value, or, where enough data exist, they show a great dispersion. So, the accuracy that we present here is not the accuracy of the reference glasses, as their “true” values are not known; instead, we show the accuracy of our results with individual studies. Where F from individual studies agree well, then these glasses are recommended as an appropriate reference material for F analyses.
Chlorine
Chlorine precision of the analyzed glasses (Table 2) ranges from 0.5 to 6.5%, with only glass BCR-2G showing a somewhat lower precision (23.5%), but with concentrations very close to the detection limit (i.e., 38 μg/g). For glasses GSE1-G (1,644 ± 9 μg/g), VG-2 (275 ± 18 μg/g), VG-568 (1,015 ± 24 μg/g), 47963 (1,261 ± 17 μg/g), VGA-99 (210 ± 7 μg/g), AC-E (303 ± 7 μg/g), GS-N (472 ± 4 μg/g), DR-N (475 ± 3 μg/g), and BHVO2-G (70 ± 4 μg/g), precision ranges from 0.5 to 6.5%. Glasses A500 (690 ± 5 μg/g) and RD5000 (880 ± 4 μg/g), from Cadoux et al. (Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017), showed a precision of 0.7 and 0.5%.
In regard to Cl (Table 3), the accuracy of our results ranges from <1% to up to 87% (Table 3) and looks independent of Cl concentrations. Overall, the accuracy, especially between our study and Zhang et al. (Reference Zhang, Koepke, Wang, Wolff, Wilke, Stechern, Almeev and Holtz2016), is very good (0.2–9%) as can be seen in Table 3 and Figure 2b. Concerning glasses A500 and RD5000 from Cadoux et al. (Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017), Cadoux (personal communication, 2020) determined the Cl content of the glasses by SIMS at 603 ± 7.5 μg/g and 762.5 ± 12.1 (errors are 2σ – SE), which is an accuracy of 14 and 15%, respectively. The highest deviation of our results between published values is observed between our results and the data from Michel & Villemant (Reference Michel and Villemant2003), for the glasses BCR-2G, GS-N, and AC-E (62, 35, and 87%, respectively). Michel & Villemant (Reference Michel and Villemant2003) used bulk methods such as pyrohydrolysis, ion chromatography, and ICP-MS to determine the Cl content of the glasses. As our EPMA data are substantially higher than the results from the bulk methods, we surmise that the glasses are heterogeneous, and that our small glass chips contain higher Cl concentrations than the bulk. We would, therefore, like to caution the reader when using these glasses are reference materials for Cl analyses of geological samples.
Bromine
As to Br, our DLs are somewhat improved, ~156 μg/g, compared with Zhang et al. (Reference Zhang, Lin, Pan, Feng, Almeev and Holtz2017), 120–300 μg/g. At the present, we cannot compare our results with natural glass reference materials, as these glasses contain only very small amounts of Br (usually less than 1 ppm), which is below the limit of detection for our EPMA method (Bureau et al., Reference Bureau, Keppler and Métrich2000; Kutterolf et al., Reference Kutterolf, Hansteen, Appel, Freundt, Krüger, Pérez and Wehrmann2013; Kendrick et al., Reference Kendrick, Arculus, Danyushevsky, Kamenetsky, Woodhead and Honda2014; Bureau, Reference Bureau and White2018). However, when we compare our EPMA results with synthetic Br-bearing glasses Cadoux et al. (Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017), we find that our method can reproduce the literature values with good accuracy (Table 4). We re-analyzed glasses A500, A1000, and RD5000 (Cadoux et al., Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017). For A500, we obtain a Br content of 409 ± 42 μg/g (precision 3.8%), that is lower to the INAA reference value (524 ± 1.6 μg/g) and similar to those measured by LA-ICP-MS and SIMS (423 ± 11 μg/g, accuracy 3%; and 381 ± 20 μg/g, accuracy 7%, respectively; Cadoux et al., Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017). Glass A1000 yielded a Br content of 1,030 ± 163 μg/g (precision 2.5%), which is consistent with the INAA reference value of 990 ± 3.2 μg/g (accuracy 3%) and similar to the contents measured by LA-ICP-MS and SIMS (1,102 ± 192 μg/g, accuracy 7%; and 1,127 ± 48 μg/g, accuracy 9%, respectively; Cadoux et al., Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017). Finally, for RD5000, we obtain a Br content of 6,512 ± 204 μg/g (precision 1.0%), which is higher than the INAA values of 5,030 ± 9 but close to the contents, within error, obtained by other in situ analyses: 5,355 ± 635 μg/g (accuracy 22%) with LA-ICP-MS and 5,638 ± 310 μg/g (accuracy 16%) with SIMS (Cadoux et al., Reference Cadoux, Iacono-Marziano, Paonita, Deloule, Aiuppa, Nelson Eby, Costa, Brusca, Berlo, Geraki, Mather, Pyle and Di Carlo2017).
Iodine
We also present a novel analytical protocol for I analyses with EPMA, with DLs between ~76 and ~150 μg/g I. Although this detection limit is probably too high for I concentrations in natural glasses (Bureau et al., Reference Bureau, Keppler and Métrich2000, Reference Bureau, Auzende, Marocchi, Raepsaet, Munsch, Testemale, Mézouar, Kubsky, Carrière, Ricolleau and Fiquet2016; Kendrick et al., Reference Kendrick, Arculus, Danyushevsky, Kamenetsky, Woodhead and Honda2014; Bureau, Reference Bureau and White2018), we can use our EPMA method to analyze experimental samples that have been doped with I. These experiments could be interesting to study I speciation in glasses, or investigate I solubility in silicate melts, or the I partitioning between crystals and melts or between immiscible melts (Steenstra et al., Reference Steenstra, van Haaster, van Mulligen, Flemetakis, Berndt, Klemme and van Westrenen2020).
New Experimental Halogen-Bearing Glasses
The good agreement of our F, Cl, and Br EPMA measurements with those of previous studies provides strong support in the use of our calibration lines to quantify halogen contents in silicate glasses.
To extend the applicability of our method, we additionally analyzed the two synthetic basaltic glasses doped with different amounts of F, Br, and I: glass SFG8 (5,000 μg/g for each halogen) and glass SFG9 (1,000 μg/g for each halogen). The calibration lines were used to subtract the “apparent” halogen contents. Sixty separate analyses were performed on each glass. The results are reported in Table 5, and the uncertainty associated with each value represents the 2σ − SΕ. Glass SFG8 has an F content of 3,592 ± 24 μg/g (precision 0.7%), Cl content of 5,098 ± 10 μg/g (precision 0.2%), Br content of 3,674 ± 23 μg/g (precision 0.6%), and I content of 5,482 ± 24 μg/g (precision 0.4%). Glass SFG9 has an F content of 529 ± 11 μg/g (precision 2.2%), Cl content of 1,187 ± 4 μg/g (precision 0.4%), Br content of 713 ± 11 μg/g (precision 1.6%), and I content of 996 ± 12 μg/g (precision 1.2%). The accuracy of our glasses can be compared only with the amount that we weighted inside. We see that our assumed values agree almost perfectly for Cl and I for all glasses (weighted amounts 5,000 and 1,000 μg/g, with accuracies of 2 and 19% for Cl, respectively; 10 and 0% for I) but not for F and Br, and that assumed values have an accuracy of 28 and 47% for F, respectively; 27 and 29%, for Br, respectively.
Concerning the in-house halogen-bearing glasses that we prepared (SFG-8 and -9), since the accuracy for Cl and I, in individual samples, is the same (Table 5), and the relative accuracy for Br and F is the same (Table 5), we can assume that the difference between the amount of halogens put in the starting mixture and the analyzed amount in the experimental samples is attributed to impurities and inhomogeneity of the chemical compounds used. In principle, no special treatment is needed for obtaining Cl contents; hence, we used the Cl concentrations to rule out potential loss of other halogens during preparation of the experimental glasses. Thus, we are confident that, by using our calibration lines, we accurately determined the F, Br, and I contents of the glasses. Moreover, the overall good precision and accuracy of our results for reference materials suggest that our in-house glasses are homogeneous and accurate. However, future studies should confirm the accuracy of our analyses with alternative analytical methods.
Analyses of Glasses in Natural Melt Inclusions
A promising application of our EPMA method lies in the analysis of the halogen contents of natural melt inclusions. To show some examples of how our method could be used, we analyzed all four halogens in olivine-hosted melt inclusions from Corvo and Pico Island in the Azores. F and Cl abundances in melt inclusions from the Pico were previously determined by EPMA by Métrich et al. (Reference Métrich, Zanon, Créon, Hildenbrand, Moreira and Marques2014). All analytical results are given in Supplementary Table 2. We compare our data with the Metrich results in Figure 3, and we find very good agreement between the two datasets for both F and Cl. Furthermore, the melt inclusion data from São Miguel (eastern Azores, Rose-Koga et al., Reference Rose-Koga, Koga, Moreira, Vlastelic, Jackson, Whitehouse, Shimizu and Habib2017, obtained by SIMS) and from Corvo (western Azores, this study) reveal compositional differences between different islands, but the reader is referred to the studies of Métrich et al. (Reference Métrich, Zanon, Créon, Hildenbrand, Moreira and Marques2014) and Rose-Koga et al. (Reference Rose-Koga, Koga, Moreira, Vlastelic, Jackson, Whitehouse, Shimizu and Habib2017) for further discussion on these matters.

Fig. 3. Fluorine versus Cl data in olivine-hosted melt inclusions from the Azores show good agreement between datasets obtained with different instruments and methods. Particularly, the two datasets obtained on melt inclusions from Pico island span the same Cl–F space. 1Present study, 2Métrich et al. (Reference Métrich, Zanon, Créon, Hildenbrand, Moreira and Marques2014), and 3Rose-Koga et al. (Reference Rose-Koga, Koga, Moreira, Vlastelic, Jackson, Whitehouse, Shimizu and Habib2017). The table with the individual data can be found in Supplementary Table 2.
Conclusions
• We produced four synthetic silicate glasses without halogens. These glasses were used to account and correct for the spectral interferences of Fe on F, Al on Br, and Ca on I peaks.
• We present a robust (high accuracy, high precision, and with improved DLs) electron microprobe method that enables the simultaneous determination of F, Br, Cl, and I contents in silicate glasses.
• We also present a novel analytical protocol for the analysis of I with EPMA in silicate glasses.
• Applying this new EPMA method, we analyzed several international reference glasses. The results show that some glasses (VG-A99, VG-2, and BCR-2G) are heterogeneous with respect to halogens and should not be used. However, other reference materials, such as VG-568, AC-E, GS-N, DR-N, and 4796, are much better suited and can be recommended for future halogen studies.
• Potential applications of this readily accessible method are demonstrated for the routine halogen analyses of experimental samples, melt inclusions, and natural glasses in a wide compositional spectrum (100's of μg/g to 1,000's of μg/g).
• Further improvements of the analytical precision will require sets of reference glasses with lower concentrations especially at the tens of μg/g to sub-μg/g level, which is the range of Br and I contents in most natural glasses.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1431927620013495.
Acknowledgments
We thank Beate Schmitte and Maik Trogisch for sample preparation and support during the electron microprobe measurements. Moreover, we also thank the members of the workshops at the Institute for Mineralogy (M. Feldhaus, J. Kemmann, P. Weitkamp, H. Heying, L. Buxtrup, and A. Gerdes) for their sterling efforts in the labs. This work was supported by the DFG (SFB-TRR 170, publ. no. 105).