Article contents
Altered Renal Pathology in an Autoimmune Disease Mouse Model After Induction of Diabetes Mellitus
Published online by Cambridge University Press: 28 May 2021
Abstract
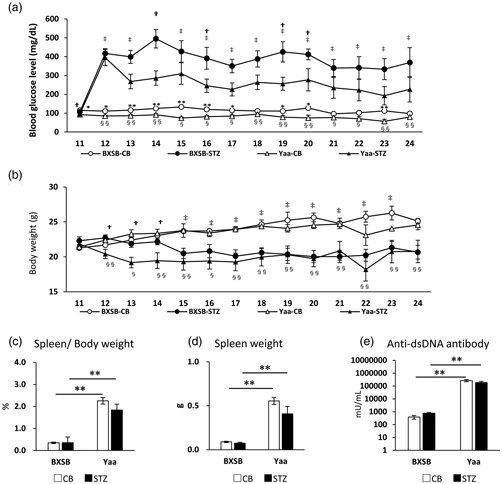
Diabetes mellitus (DM) is a predisposing factor for renal disorder progression and is referred to as diabetic kidney disease (DKD). However, there are no reports of DKD with an underlying autoimmune disorder. In this study, we compared the pathophysiological changes caused by DM induction after streptozotocin (STZ) injection in comparison with that in a control group receiving citrate buffer (CB) in the autoimmune disease model mice “BXSB/MpJ-Yaa” (Yaa) and the wild-type strain BXSB/MpJ. Both strains showed hyperglycemia after 12 weeks of STZ injection. Interestingly, the Yaa group developed membranous and proliferative glomerulonephritis, which tended to be milder glomerular lesions in the STZ group than in the CB group, as indicated by a decreased mesangial area and ameliorated albuminuria. Statistically, the indices for hyperglycemia and autoimmune abnormalities were negatively and positively correlated with the histopathological parameters for mesangial matrix production and glomerular proliferative lesions, respectively. STZ treatment induced renal tubular anisonucleosis and dilations in both strains, and they were more severe in Yaa. Significantly decreased cellular infiltration was observed in the Yaa group compared to the CB group. Thus, in DKD related to autoimmune nephritis, hyperglycemia modifies its pathology by decreasing the mesangial area and interstitial inflammation and aggravating renal tubular injury.
- Type
- Biological Applications
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 1
- Cited by



