Article contents
Simultaneous Three-Dimensional Vascular and Tubular Imaging of Whole Mouse Kidneys With X-ray μCT
Published online by Cambridge University Press: 06 July 2020
Abstract
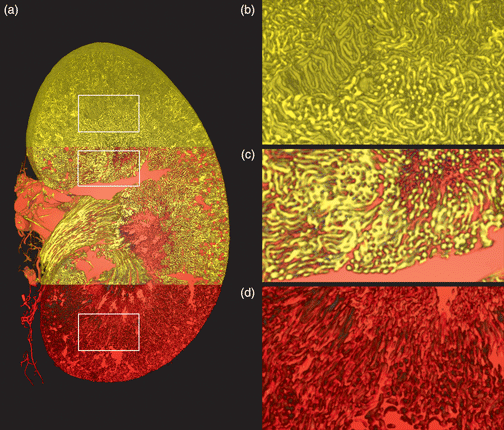
Concurrent three-dimensional imaging of the renal vascular and tubular systems on the whole-kidney scale with capillary level resolution is labor-intensive and technically difficult. Approaches based on vascular corrosion casting and X-ray micro computed tomography (μCT), for example, suffer from vascular filling artifacts and necessitate imaging with an additional modality to acquire tubules. In this work, we report on a new sample preparation, image acquisition, and quantification protocol for simultaneous vascular and tubular μCT imaging of whole, uncorroded mouse kidneys. The protocol consists of vascular perfusion with the water-soluble, aldehyde-fixable, polymeric X-ray contrast agent XlinCA, followed by laboratory-source μCT imaging and structural analysis using the freely available Fiji/ImageJ software. We achieved consistent filling of the entire capillary bed and staining of the tubules in the cortex and outer medulla. After imaging at isotropic voxel sizes of 3.3 and 4.4 μm, we segmented vascular and tubular systems and quantified luminal volumes, surface areas, diffusion distances, and vessel path lengths. This protocol permits the analysis of vascular and tubular parameters with higher reliability than vascular corrosion casting, less labor than serial sectioning and leaves tissue intact for subsequent histological examination with light and electron microscopy.
- Type
- Biological Applications
- Information
- Copyright
- Copyright © Microscopy Society of America 2020
Footnotes
These authors contributed equally to the present study.
References
- 6
- Cited by



