Published online by Cambridge University Press: 26 November 2021
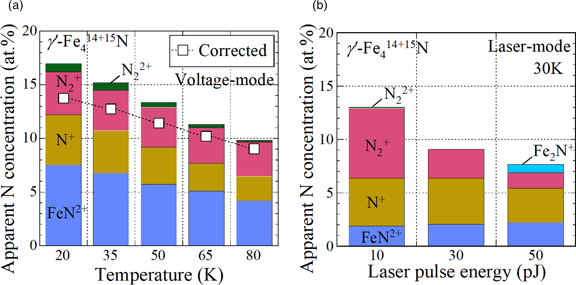
The nitrogen deficiency in steels measured by atom probe tomography (APT) is considered to arise from the obscurement of singly charged dimer nitrogen ions (N2+) by the iron-dominant peak (56Fe2+) at 28 Da. To verify this by quantifying the amount of N2+ ions, γ′-Fe4N consisting of the 15N isotope was prepared on iron substrates by plasma nitriding using a nitrogen isotopic gas (15N2). Although considerable amounts of 15N2+ were observed at 30 Da without overlap with any iron peak, the observed nitrogen concentrations of γ′-Fe4N were clearly lower than the stoichiometric composition (19–20 at%), using both pulsed voltage and pulsed laser atom probes. The origin of the missing nitrogen, excluding nitrogen obscured by other ion species, was predicted to be the occurrence of neutral nitrogen or nitrogen gas molecules in field evaporation. The generation rate of iron nitride ions (FeN2+) for 15N was significantly lower than that for 14N in γ′-Fe4N, which affected the amount of the missing nitrogen. The isotope effect suggests that the isotopic ratio cannot always be determined from only one ion species among the multiple species observed in the APT analysis. We discuss the mechanism of the isotope effect in FeN2+ formation by field evaporation.