Article contents
Morphological Changes in the Myotendinous Junction of mdx Mice
Published online by Cambridge University Press: 11 August 2021
Abstract
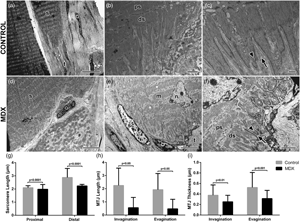
The myotendinous junction (MTJ) is the interface between muscle and tendon, and it is the main area of force transmission of the locomotor apparatus. Dystrophic processes promote pathological injury which affects the skeletal muscle and can influence the morphology of the MTJ. This study aimed to investigate the adaptations in MTJ morphology of mdx mice in the tibialis anterior muscle. Male mice (n = 24) were divided into Control—C57bl/10 and mdx—C57bl/10mdx (Duchenne muscular dystrophy experimental model). In the mdx group, centralized nuclei with a large area and greater deposition of type III collagen (fibrosis) were observed. Also, shorter sarcomeres and sarcoplasmatic projections of MTJ were observed. We concluded that the adaptations in mdx mice demonstrated extensive impairment in the MTJ region with reduced ultrastructures.
- Type
- Micrographia
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 5
- Cited by



