Published online by Cambridge University Press: 07 October 2021
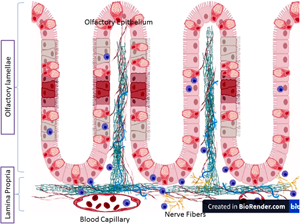
Piscine mast cells or eosinophilic granule cells (EGCs) of fish are equivalent to the mammalian mast cells. Recently, a better understanding of EGCs functions is allowed because of the growing interest in fish models. Herein, we present a trial to furnish data regarding the distribution of the EGCs in the fish olfactory organ, an issue that has not been reported so far. Regarding their distribution, two kinds of EGCs had been identified. An intra-epithelial one was detected in the olfactory epithelium lining of the olfactory lamellae. The stromal one was identified in the connective tissue core of the olfactory lamellae and in the lamina propria underlying the olfactory epithelium. Some were detected in the capillary lumen. The cytoplasm of the EGCs reacted strongly with the MMP-9 antibody. Stimulating a migration perspective for the olfactory EGCs which was confirmed by their location in the blood capillaries. Several EGCs could be verified in close relation, some underneath the epineurium, with the nerve fiber. Mutually, this verifies the existence of intra-epithelial and stromal migrating EGCs in the catfish olfactory organ and their inclusion in the olfactory immune response. Additionally, this provides evidence for an immune–nervous interaction to influence both the immune reactions and the nervous scheme in catfish.
Current address: Department of Developmental Biology, Graduate School of Integrated Science for Life, Hiroshima University, Higashi-Hiroshima City 739-8526, Japan.