No CrossRef data available.
Article contents
Microstructural SEM/STEM Analysis of Ni70Ga30 and Ni70Sn30 Catalytically Active Intermetallic Ribbons after Prolonged Exposure to Elevated Process Temperature
Published online by Cambridge University Press: 30 July 2021
Abstract
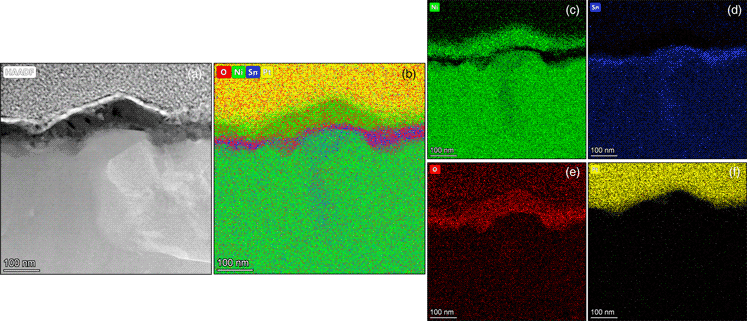
Melt-spun Ni70Ga30 and Ni70Sn30 monolithic catalysts, when subjected to prolonged oxidation in air at 770 K, undergo different microstructural changes. Whereas Ni70Ga30 is largely stable and composed of Ni3Ga phase, the Ni70Sn30 develops a two-layer microstructure on the exposed ribbon surface. The two-layer structure consists of a Ni-rich zone, which is founded on a SnO2 sublayer. In the bulk, the Ni70Sn30 ribbon is heterogeneous in phase composition and comprises of Ni3Sn and Ni3Sn2 phases. The development of Ni particulates on the outer ribbon surface, through simple oxidation heat treatment, may thus be a way for improving the catalytic performance of Ni70Sn30 ribbons, resembling that of Ni3Al ribbons spontaneously activated under reaction conditions.
- Type
- The XVIIth International Conference on Electron Microscopy (EM2020)
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America


