Published online by Cambridge University Press: 10 February 2022
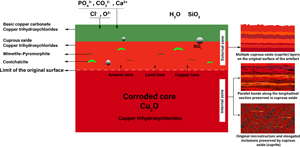
The morphology and composition of the corrosion products of archaeological arsenical copper alloys buried in a specific environment for a long time were investigated using optical microscopy (OM), scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDS), micro X-ray fluorescence (μ-XRF), and X-ray diffraction (XRD). The analyses demonstrated that the alloy composition of the artifacts was copper-arsenic (Cu-AS) with significant amounts of lead in some samples. Cuprite, malachite, and copper (II) hydroxychlorides were observed on a completely mineralized matrix. The surface of the objects was covered by two main corrosion layers formed on the internal cuprous oxide. However, several successive layers of corrosion products were recognized on the artifacts in some cases. Furthermore, phosphatic corrosion products including, mimetite [Pb5(AsO4)3Cl], and pyromorphite [Pb5(PO4)3Cl] were identified. Conichalcite [CaCu(AsO4)](OH), which grew in the form of Liesegang rings, was also identified as a corrosion product of one of the objects. As and Pb exhibited some enrichment in the corrosion crusts of the artifacts. Environmental changes in burial conditions, particularly due to seasonal streams and consequent changes in soil corrosivity, vicinity of objects to bone material, along with the migration of alloy elements, especially lead and arsenic, can explain the morphological features of these archaeological objects.