Article contents
Improving the Quantification of Deuterium in Zirconium Alloy Atom Probe Tomography Data Using Existing Analysis Methods
Published online by Cambridge University Press: 01 October 2021
Abstract
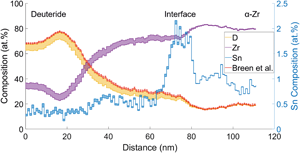
Zirconium alloys are common fuel claddings in nuclear fission reactors and are susceptible to the effects of hydrogen embrittlement. There is a need to be able to detect and image hydrogen at the atomic scale to gain the experimental evidence necessary to fully understand hydrogen embrittlement. Through the use of deuterium tracers, atom probe tomography (APT) is able to detect and spatially locate hydrogen at the atomic scale. Previous works have highlighted issues with quantifying deuterium concentrations using APT due to complex peak overlaps in the mass-to-charge-state ratio spectrum between molecular hydrogen and deuterium (H2 and D). In this work, we use new methods to analyze historic and simulated atom probe data, by applying currently available data analysis tools, to optimize solving peak overlaps to improve the quantification of deuterium. This method has been applied to literature data to quantify the deuterium concentrations in a concentration line profile across an α-Zr/deuteride interface.
Keywords
- Type
- Detection of Hydrogen
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 4
- Cited by



