Published online by Cambridge University Press: 23 December 2021
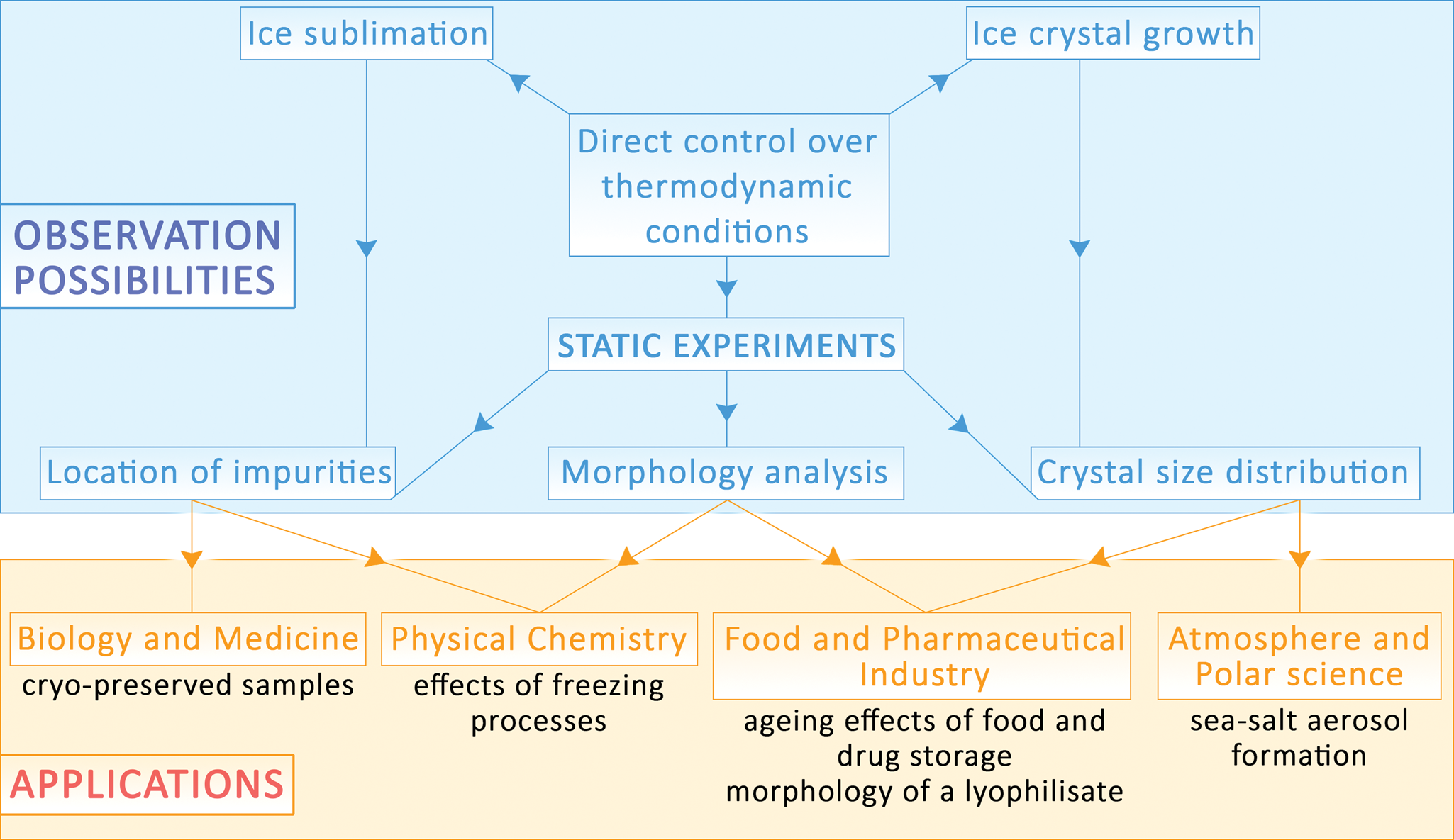
Frozen aqueous solutions are an important subject of study in numerous scientific branches including the pharmaceutical and food industry, atmospheric chemistry, biology, and medicine. Here, we present an advanced environmental scanning electron microscope methodology for research of ice samples at environmentally relevant subzero temperatures, thus under conditions in which it is extremely challenging to maintain the thermodynamic equilibrium of the specimen. The methodology opens possibilities to observe intact ice samples at close to natural conditions. Based on the results of ANSYS software simulations of the surface temperature of a frozen sample, and knowledge of the partial pressure of water vapor in the gas mixture near the sample, we monitored static ice samples over several minutes. We also discuss possible artifacts that can arise from unwanted surface ice formation on, or ice sublimation from, the sample, as a consequence of shifting conditions away from thermodynamic equilibrium in the specimen chamber. To demonstrate the applicability of the methodology, we characterized how the true morphology of ice spheres containing salt changed upon aging and the morphology of ice spheres containing bovine serum albumin. After combining static observations with the dynamic process of ice sublimation from the sample, we can attain images with nanometer resolution.