Article contents
Determination of the Chemical Compositions of Fine titanium Carbide and Niobium Carbide Precipitates in Isothermally Aged Ferritic Steel by Atom Probe Tomography Analysis
Published online by Cambridge University Press: 07 December 2020
Abstract
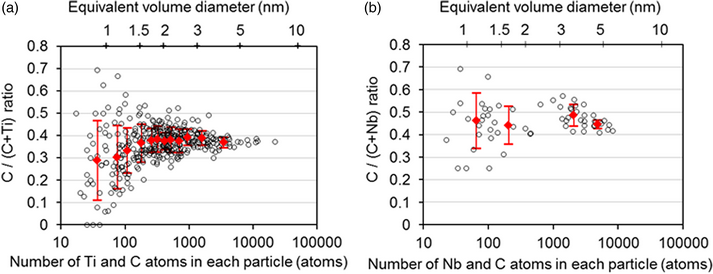
The carbon (C) ratios, namely the atomic ratios of C/(C + M), in nano-sized coherent MC precipitates (M = Ti, Nb) with the NaCl-type (B1) structure in ferritic steels, which had been isothermally aged at 580 °C, were investigated using atom probe tomography (APT). Considering the influences of the trajectory aberration, detection loss, and peak overlap, we determined the C ratios to be ~0.40 and ~0.45 for an equivalent volume diameter of 1.5–5 nm and 1–5 nm for the TiC and NbC precipitates, respectively, suggesting that there is a considerable fraction of C vacancies in both nano-sized precipitates. The apparent C ratios show significant scatter with decreasing particle size, while the apparent mean C ratios of very fine TiC particles, smaller than 1.5 nm, decreased with decreasing particle size. With the use of one of the latest APT instruments with a high detection efficiency, the scattering in the apparent C ratios was reduced because the counting statistics were improved; however, the artificial enrichment of C atoms to particular crystallographic directions of ferrite hindered the determination of the C ratio for very fine TiC particles smaller than 1.5 nm.
- Type
- Materials Science Applications
- Information
- Copyright
- Copyright © The Author(s), 2020. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 6
- Cited by



