Article contents
Al2O3 Grain Boundary Segregation in a Thermal Barrier Coating on a Ni-Based Superalloy
Published online by Cambridge University Press: 13 May 2022
Abstract
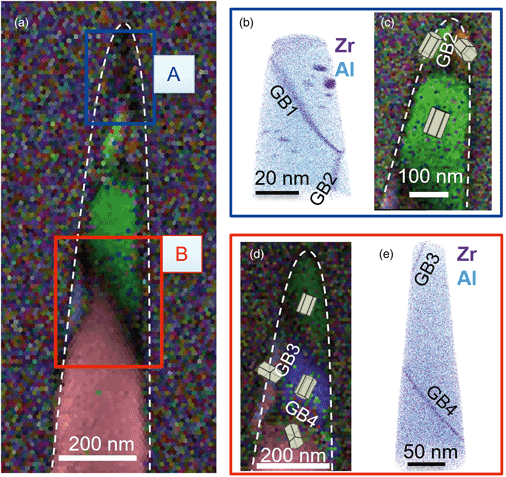
The segregation of reactive elements (REs) along thermally grown oxide (TGO) grain boundaries has been associated to slower oxide growth kinetics and improved creep properties. However, the incorporation and diffusion of these elements into the TGO during oxidation of Ni alloys remains an open question. In this work, electron backscatter diffraction in transmission mode (t-EBSD) was used to investigate the microstructure of TGO within the thermal barrier coating on a Ni-based superalloy, and atom probe tomography (APT) was used to quantify the segregation behavior of REs to α-Al2O3 grain boundaries. Integrating the two techniques enables a higher level of site-specific analysis compared to the routine focused ion beam lift-out sample preparation method without t-EBSD. Needle-shaped APT specimens readily meet the thickness criterion for electron diffraction analysis. Transmission EBSD provides an immediate feedback on grain orientation and grain boundary location within the APT specimens to help target grain boundaries in the TGO. Segregation behavior of REs is discussed in terms of the grain boundary character and relative location in TGO.
Keywords
- Type
- Materials Science Applications
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 3
- Cited by


