INTRODUCTION
The Hawaiian Islands are part of the geographically longest and geologically oldest volcanic chains on Earth. Extensive coral reefs exist on every island and shallow bank of the archipelago, from the island of Hawai‘i, past Kure Atoll, over 2000 miles to the north-west. The vast geographic isolation of the Hawaiian Archipelago has produced a unique biodiversity that is marked by one of the highest levels of marine endemism recorded on Earth (DeMartini & Friedlander, Reference DeMartini and Friedlander2004; Kane et al., Reference Kane, Kosaki and Wagner2014). While Hawai‘i's terrestrial and shallow-water (40 m) marine biodiversity have been well surveyed and documented (Eldredge & Evenhuis, Reference Eldredge and Evenhuis2003; Eldredge, Reference Eldredge2006), organisms that inhabit Hawai‘i's deeper marine ecosystems remain only marginally explored (Baco, Reference Baco2007; Parrish & Baco, Reference Parrish, Baco, Lumsden, Hourigan, Bruckner and Dorr2007). Antipatharians, commonly known as black corals, represent one of such particularly undersurveyed taxonomic groups, as evidenced by the high rates of species discoveries from recent deep-water surveys around the Hawaiian Islands (Opresko, Reference Opresko2003, Reference Opresko2005; Baco, Reference Baco2007; Parrish & Baco, Reference Parrish, Baco, Lumsden, Hourigan, Bruckner and Dorr2007).
The Antipatharia is an anthozoan order within the subclass Hexacorallia, encompassing seven families, 43 genera and over 235 species (Wagner et al., Reference Wagner, Luck and Toonen2012). The order is characterized by: (1) a skeleton that is primarily proteinaceous and covered with minute skeletal spines; (2) polyps with six unbranched tentacles that are non-retractile; (3) six primary mesenteries; and (4) exclusively colonial organisms (Wagner et al., Reference Wagner, Luck and Toonen2012). Black corals occur worldwide in all oceans from polar to tropical regions, and have a wide depth distribution ranging from 2 m for tropical wire corals, down to abyssal depths of over 8600 m for species in the Western Pacific (reviewed by Wagner et al., Reference Wagner, Luck and Toonen2012). Despite this wide bathymetric range, black corals are primarily found in deep waters below the photic zone, with over 75% of described species occurring below 50 m (Cairns, Reference Cairns2007). At these depths, black corals are often abundant and dominant components of the sessile invertebrate fauna, and create a habitat for a myriad of species and associates (reviewed by Wagner et al., Reference Wagner, Luck and Toonen2012). However, because most species are found below the depth limits of conventional SCUBA diving, very little is known about the basic biology and ecology of black corals.
In Hawai‘i black corals are of particular importance, not just from an ecological perspective, but also from a cultural and economic one. First, black corals are dominant habitat-forming species on Hawaiian deep reefs (>50 m) (Baco, Reference Baco2007; Parrish & Baco, Reference Parrish, Baco, Lumsden, Hourigan, Bruckner and Dorr2007). Second, black corals are culturally important as they represent the official gemstone of the State of Hawai‘i and were traditionally used in Hawaiian culture for medicinal purposes (Kaaiakamanu & Akina, Reference Kaaiakamanu and Akina1922; Chun, Reference Chun1994). Third, Hawai‘i is the only place in the United States, and only one of few places in the world, where black coral is harvested commercially for the precious coral jewellery industry, which is supplied by SCUBA divers that collect the species Antipathes griggi Opresko, 2009, Antipathes grandis Verril, 1928 and Myriopathes cf. ulex (Ellis and Solander, 1786) at depths between 40 and 70 m (reviewed by Grigg, Reference Grigg2001). As a result of collaborations between scientists and the Hawaiian black coral fishery, antipatharian populations have been well documented in Hawai‘i, especially in comparison to most other geographic locations (Wagner et al., Reference Wagner, Luck and Toonen2012). Consequently, a large collection of specimens and records exists for this group in Hawai‘i. However, because of taxonomic uncertainties, most previous surveys have not examined ecological features to the level of individual species. The purpose of this study was to map populations of Hawaiian shallow-water (<150 m) antipatharian species, which is a first order approach to revealing ecological differences between species. Specifically, data on geographic position, depth, substrate type and temperature were analysed in relation to species, in order to determine whether there are differences in these habitat parameters between different antipatharian species. The depth zone examined as part of this study included mesophotic coral ecosystems (MCEs), which are light-dependent coral reefs and associated organisms found below the depth limits of conventional SCUBA diving (30 m), and extending to depths of 150 m in regions with high water clarity like Hawai‘i (Kahng et al., Reference Kahng, Garcia-Sais, Spalding, Brokovich, Wagner, Weil, Hinderstein and Toonen2010). MCEs are notoriously undersurveyed worldwide due to the logistical challenges of accessing areas below the depth limits of conventional SCUBA diving.
MATERIALS AND METHODS
Antipatharian records from Hawaiian waters were retrieved from: (1) the literature; (2) museum specimens; (3) recently collected specimens; and (4) archived video/photo records. Only records <150 m were retrieved, because this depth is considered the depth limit of MCEs in the Hawaiian Islands (Kahng et al., Reference Kahng, Garcia-Sais, Spalding, Brokovich, Wagner, Weil, Hinderstein and Toonen2010) and because records below this depth were far scarcer and more uncertain. Literature records were obtained from over 400 articles that were collected as part of a literature review on the biology and ecology of black corals (Wagner et al., Reference Wagner, Luck and Toonen2012). Examined museum specimens included all Hawaiian black corals deposited at the Bernice P. Bishop Museum (BPBM) in Honolulu, Hawai‘i, and the National Museum of Natural History, Smithsonian Institution in Washington, DC (USNM). Recently collected specimens were obtained on a series of expeditions throughout the Hawaiian Archipelago between 2006 and 2014 using SCUBA, mixed-gas technical diving and the Hawai‘i Undersea Research Laboratory (HURL) manned submersibles Pisces IV and V. Video/photo records were acquired from Hawaiian deep-sea explorations conducted by HURL and the Monterrey Bay Aquarium Research Institute (MBARI), and included dives conducted by the manned submersibles Makali‘i, Pisces IV and Pisces V, as well as the remotely operated vehicles RCV-150 and Tiburon. Each black coral record was mapped, and the following information was recorded where available: (1) species; (2) latitude/longitude; (3) depth; (4) in situ temperature; and (5) substrate type. In cases where species assignments could not be made from video/photo records alone, identifications were made using specimens collected in surrounding areas. Temperature data was only available for HURL and MBARI records, and was retrieved from the CTD data recorder of the respective underwater vehicle at the time black coral colonies were collected or photographed.
RESULTS
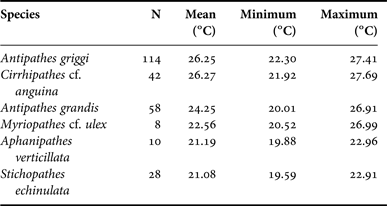
The Hawaiian Archipelago has been extensively surveyed for antipatharians, with individual surveys targeting areas from Hawai‘i Island to Kure Atoll; however, the vast majority of historical surveys are restricted to the inhabited Main Hawaiian Islands (Figure 1). A total of 862 individual black coral records were available from waters surrounding the Hawaiian Islands at depths ranging between 9 and 150 m (Figure 1). Archived video/photo records from HURL provided the largest proportion of records (N = 332), followed by recent specimen collections (N = 258), literature records (N = 143), USNM museum specimens (N = 113), BPBM museum specimens (N = 12) and MBARI records (N = 4) (Figure 1). All black corals were exclusively recorded on hard substrates. Some colonies were located within close proximity to patches of sand; however, black corals were always firmly anchored to hard substrates. Eight different species were identified among the reviewed records, and included: (1) Antipathes griggi; (2) Antipathes grandis; (3) Cirrhipathes cf. anguina (Dana, 1846); (4) Stichopathes echinulata Brook, 1889; (5) Stichopathes? sp.; (6) Aphanipathes verticillata Brook, 1889; (7) Acanthopathes undulata (Van Pesch, 1914); and (8) Myriopathes cf. ulex (Figure 2; see Wagner, Reference Wagner2011 for detailed descriptions). All antipatharian species were recorded from overlapping locations within the Hawaiian Islands, although there were differences in the geographic spread of individual species along the archipelago (Figure 3). Most species were widely distributed along the Hawaiian Islands, including Antipathes griggi from Hawai‘i to Pearl and Hermes Atoll, Antipathes grandis from Hawai‘i to Ni‘ihau, C. cf. anguina from Hawai‘i to north-west of Brooks Banks, Stichopathes echinulata from Hawai‘i to Lisianski, Stichopathes? sp. from Hawai‘i to French Frigate Shoals, Acanthopathes undulata from Hawai‘i to Laysan and M. cf. ulex from Hawai‘i to Pearl and Hermes Atoll (Figure 3). In contrast, Aphanipathes verticillata was recorded only from recent collections (2008–2009) performed in the Keyhole Pinnacle area of the Au‘au Channel (Figure 3). In terms of bathymetric distributions, all antipatharian species were found at overlapping depths (Figure 4), although there were significant differences in the mean depth between species one-way ANOVA P < 0.0001). Within the examined depth range (0–150 m), Stichopathes? sp. had the shallowest depth distribution (mean = 30.0 m, range = 9–58 m), followed by Antipathes griggi (mean = 48.9 m, range = 9–110 m), C. cf. anguina (mean = 51.0 m, range = 9–150 m), Antipathes grandis (mean = 83.3 m, range = 24–146 m), M. cf. ulex (mean = 85.7 m, range = 30–150 m), Acanthopathes undulata (mean = 90.0 m, range = 32–150 m), Aphanipathes verticillata (mean = 105.5 m, range = 88–130 m) and Stichopathes echinulata (mean = 124.8 m, range = 90–150 m) (Figure 4). In most cases, 95% confidence intervals of mean depths did not overlap between species, with the exception of Antipathes griggi overlapping with C. cf. anguina, and Antipathes grandis overlapping with M. cf. ulex. In terms of temperature, records were only available for six of the eight species identified within the examined depth range (0–150 m) (Table 1). As with depth, mean temperatures were significantly different between species (one-way ANOVA P < 0.0001), a pattern that is expected given the general correlation between temperature and depth. Antipathes griggi was recorded at the warmest temperatures, followed by C. cf. anguina, Antipathes grandis, M. cf. ulex, Aphanipathes verticillata, Stichopathes echinulata and Acanthopathes undulata (Table 1). In most cases, 95% confidence intervals of mean temperatures did not overlap between species, with the exception of Antipathes griggi overlapping with C. cf. anguina, and Aphanipathes verticillata overlapping with M. cf. ulex and Stichopathes echinulata.

Fig. 1. Map showing the locations of shallow-water (<150 m) antipatharian records examined as part of this study. Black coral records include video/photo records from HURL and MBARI, museum specimens deposited at the USNM and BPBM, and recently collected specimens (N = 862).

Fig. 2. In situ photographs of Hawaiian shallow-water (<150 m) antipatharian species. (A). Antipathes griggi, (B) Antipathes grandis, (C) Cirrhipathes cf. anguina, (D) Stichopathes echinulata, (E) Stichopathes? sp., (F) Aphanipathes verticillata, (G) Acanthopathes undulata and (H) Myriopathes cf. ulex (photographs courtesy of HURL).

Fig. 3. Map showing the spatial distribution of Hawaiian shallow-water (<150 m) black coral species. (A) Antipathes griggi, (B) Antipathes grandis, (C) Cirrhipathes cf. anguina, (D) Stichopathes echinulata, (E) Stichopathes? sp., (F) Aphanipathes verticillata, (G) Acanthopathes undulata and (H) Myriopathes cf. ulex.

Fig. 4. Hawaiian shallow-water (<150 m) black coral records by depth and species. Depths represent upper bin limits. Records include published reports from the literature, museum specimens deposited at the USNM and BPBM, specimens collected on a series of expeditions throughout the archipelago in 2006–2014 and archived video/photo records from HURL and MBARI (N = 862).
Table 1. Temperature data by species for Hawaiian shallow-water (<150 m) black corals. Data was retrieved from the CTD recorder of the underwater vehicle at the time colonies were photographed or collected. Temperature records were only available for six of the eight antipatharian species identified within the examined depth range.
DISCUSSION
Studies on biogeographical distributions are very rare within the order Antipatharia, because the vast majority of species are known only from their type locality and consequently have limited geographic ranges (Wagner et al., Reference Wagner, Luck and Toonen2012). This is due to taxonomic uncertainties within the Antipatharia, as well as the lack of historical surveys focusing on this taxonomic order. That said, some geographic locations have been relatively well surveyed for antipatharians, including the Gulf of Mexico, the Mediterranean and the Hawaiian Archipelago (reviewed by Wagner et al., Reference Wagner, Luck and Toonen2012). In fact, antipatharian populations from the Hawaiian Islands are some of the best documented on the globe (Wagner et al., Reference Wagner, Luck and Toonen2012). This is due, in large part, to a commercial black coral fishery that has operated in Hawai‘i since the late 1950s (reviewed by Grigg, Reference Grigg2001) and has led to many surveys across the archipelago. However, the vast majority of these surveys are restricted to the inhabited Main Hawaiian Islands (Figure 1), and in particular to the shores of Maui, Kaua‘i and Hawai‘i, which are home to the largest populations of commercially valuable Hawaiian black corals in the state (Grigg, Reference Grigg2001). Records of black corals from the North-western Hawaiian Islands are far scarcer, because fewer surveys have targeted those areas (Wagner et al., Reference Wagner, Papastamatiou, Kosaki, Gleason, McFall, Boland, Pyle and Toonen2011). The only survey that has quantified population densities of antipatharians in the North-western Hawaiian Islands notes densities of up to 0.047 colonies m−2 for Antipathes griggi and 0.690 colonies m−2 for Myriopathes cf. ulex (Wagner et al., Reference Wagner, Papastamatiou, Kosaki, Gleason, McFall, Boland, Pyle and Toonen2011). In contrast, densities exceeding 1 colony m−2 exist at several locations throughout the Main Hawaiian Islands for these two species (Grigg, Reference Grigg2001). Future surveys targeting reefs in the North-western Hawaiian Islands will be needed to determine whether black corals are less abundant there.
The results of this study indicate that Hawaiian shallow-water antipatharian species are widely distributed across the archipelago, with the possible exception of Aphanipathes verticillata, which to date has only been recorded in Hawaii from the Au‘au Channel (Figure 3). However, Aphanipathes verticillata superficially resembles Antipathes griggi in terms of colony morphology and, therefore, distinguishing these two species on videos, photographs or in situ is very difficult (Figure 2; Opresko et al., Reference Opresko, Wagner, Montgomery and Brugler2012). Consequently, the narrow range of Aphanipathes verticillata may be because this species has been misidentified as Antipathes griggi in the past. Besides the Au‘au Channel, Aphanipathes verticillata is also known from Mauritius and Okinawa, although the Hawaiian form is considered a distinct subspecies (Aphanipathes verticillata mauiensis) due to the unique morphological features of its skeletal spines (Opresko et al., Reference Opresko, Wagner, Montgomery and Brugler2012). Mauritius, Okinawa and Hawai‘i are all separated by large geographic distances, which suggests that Aphanipathes verticillata is also likely to be present in other locations, including within the Hawaiian Islands. Future surveys will be needed to verify whether Aphanipathes verticillata has a limited distribution within the Hawaiian Archipelago.
Based on all available records, Hawaiian shallow-water antipatharian species coexist on hard substrates within similar geographic, depth and temperature ranges (Figure 3). These results are consistent with previous antipatharian surveys in other parts of the globe, which note that multiple black coral species typically coexist sympatrically (Goenaga, Reference Goenaga1977; Warner, Reference Warner1981; Grange & Singleton, Reference Grange and Singleton1988; Oakley, Reference Oakley1988; Sanchez et al., Reference Sanchez, Zea and Diaz1998; Sanchez, Reference Sanchez1999; Tazioli et al., Reference Tazioli, Bo, Boyer, Rotinsulu and Bavestrello2007). Collectively, these observations indicate that there are generalities in the physical habitat requirements of shallow-water (<150 m) antipatharians. Specifically, previous studies note that almost all black corals require hard substrates for attachment, and are often found in areas with strong currents and low-light levels (reviewed by Wagner et al., Reference Wagner, Luck and Toonen2012). The principle of competitive exclusion predicts that for multiple species to coexist, they must be unique in terms of their microhabitat or use of resources (Gause, Reference Gause1932; Hardin, Reference Hardin1960). The results of this study indicate that there are several similarities in the habitats of all Hawaiian shallow-water (<150 m) antipatharian species, including the need for hard substrates and overlapping geographic, bathymetric and temperature distributions. In most cases, however, individual species appear to be specialized in exploiting a particular microhabitat in terms of depth and temperature (Figure 4; Table 1), parameters which are themselves interrelated. In contrast, individual species are not differentiated by substrate type, as all species were exclusively recorded on hard substrates. The requirement for hard substrate appears to be generally true across the order Antipatharia, with the exception of species within the genus Schizopathes, which have a modified, hook-like holdfast for support on soft bottoms (Opresko, Reference Opresko1997, Reference Opresko2002). Apart from this deep-water genus (>750 m), all other antipatharians possess a basal plate that is firmly anchored to hard substrates.
There are large overlaps in the bathymetric distribution between Hawaiian antipatharian species (Figure 4). For example, the species Antipathes griggi, Antipathes grandis, Cirrhipathes cf. anguina, Stichopathes sp.?, Acanthopathes undulata and M. cf. ulex are all present within depths accessible through conventional SCUBA diving (40 m), whereas Aphanipathes verticillata and Stichopathes echinulata do not start appearing until depths below 80 m (Figure 4). Despite these large overlaps in the depth distribution of individual species, each species appears to have a preferred depth range which they exploit (Figure 4). The mean depth was significantly different for each species, with the exception of no statistically significant differences between Antipathes griggi and C. cf. anguina, and Antipathes grandis and M. cf. ulex. Similar to depth distributions, mean temperatures were significantly different between species, with the exception of no significant differences between Antipathes griggi and C. cf. anguina, and M. cf. ulex and Stichopathes echinulata. Interestingly, in cases in which species have overlapping mean depths and temperatures, the involved species have very different morphologies (Figure 2). Antipathes griggi has a bushy, irregularly branched corallum, whereas C. cf. anguina is an unbranched wire-coral (Figure 2). Similarly, Antipathes grandis has a bushy corallum, M. cf. ulex has fan-shaped colonies and Stichopathes echinulata is an unbranched wire coral (Figure 2). Furthermore, polyp sizes are significantly different between each pair of species with overlapping depth ranges (see Wagner, Reference Wagner2011). Thus, although Antipathes griggi and C. cf. anguina, Antipathes grandis and M. cf. ulex, and M. cf. ulex and Stichopathes echinulata inhabit very similar habitats in the Hawaiian Islands, they may avoid competition by exploiting different resources through their distinct morphologies and polyp sizes. Specifically, different colony morphologies and polyp sizes may allow species to specialize on feeding most efficiently under a particular current flow regime. While current regime was not analysed as part of this study, both current direction and speed have been shown to be important in shaping the spatial distribution of sessile suspension feeders (Chamberlain & Graus, Reference Chamberlain and Graus1975; Baynes & Szmant, Reference Baynes and Szmant1989). Unfortunately, data sets for these two variables are not available across the survey areas examined as part of this study, and consequently future studies will have to examine whether there are differences in these variables between the environmental ranges of black coral species. In relation to current flow regime, previous studies note that in areas where current direction is primarily uni-directional, fan-shaped antipatharians are common and oriented perpendicular to currents; an orientation that maximizes contact between feeding surfaces and suspended food (reviewed by Wagner et al., Reference Wagner, Luck and Toonen2012). Future studies will need to examine whether black coral species specialize on exploiting particular microenvironments in terms of current direction and speed, but the observed morphological differences between the species examined as part of this study indicate that currents may be important in shaping their spatial distributions.
Another interesting feature in the depth distributions of Hawaiian antipatharians is that at least some species appear to be mostly limited to depths shallower than 120 m (Figure 4), an observation that is consistent with previous surveys of Hawaiian antipatharians (Grigg, Reference Grigg2001; Kahng & Grigg, Reference Kahng and Grigg2005; Wagner et al., Reference Wagner, Brugler, Opresko, France, Montgomery and Toonen2010). The depth of 120 m corresponds to the top of the thermocline in the Main Hawaiian Islands (Grigg et al., Reference Grigg, Polovina, Friedlaner, Rohmann, Riegl and Dodge2008), suggesting that temperature may be an important factor in limiting the bathymetric distributions of some black coral species. While this correlation does not necessarily signify a causal relationship, temperature is thought to be one of the main factors limiting distributions of ectothermic organisms at high latitudes, as well as in deep water (Pörtner, Reference Pörtner2002; Grigg, Reference Grigg2006). Exposures to low temperatures have been shown to adversely affect feeding, reproduction and growth of corals (Palardy et al., Reference Palardy, Grottoli and Matthews2005; Putnam et al., Reference Putnam, Edmunds and Fan2008; De Putron & Ryland, Reference De Putron and Ryland2009), and in more severe cases cause large-scale mortalities (Roberts et al., Reference Roberts, Rouse, Walker and Hudson1982; Coles & Fadlallah, Reference Coles and Fadlallah1991; Laboy-Nieves et al., Reference Laboy-Nieves, Klein, Conde, Losada, Cruz and Bone2001; Hoegh-Guldberg et al., Reference Hoegh-Guldberg, Fine, Skirving, Johnstone, Dove and Strong2005). The absence of some black coral species below the top of the thermocline in the Main Hawaiian Islands (120 m; Figure 4) suggests that they may be negatively affected by temperature. The top of the thermocline is found at shallower depths in the North-western Hawaiian Islands, shoaling to depths of 60 m at Pearl and Hermes Atoll (Grigg et al., Reference Grigg, Polovina, Friedlaner, Rohmann, Riegl and Dodge2008). Interestingly, the only black coral survey that has targeted reefs at depths between 50 and 80 m at Pearl and Hermes Atoll did not record any black corals below 60 m (Wagner et al., Reference Wagner, Papastamatiou, Kosaki, Gleason, McFall, Boland, Pyle and Toonen2011). Even though these observations are very limited, they suggest that low temperatures may set a lower depth limit for some black coral species. In this regard, in situ temperatures recorded for the various species as part of this study (Table 1) may aid in designing future controlled experiments to test for the effects of temperature on black coral survival.
Although the Hawaiian Archipelago is one of the best surveyed areas for antipatharians on the planet, some areas, such as the uninhabited North-western Hawaiian Islands, still remain only marginally explored. The initial surveys across the Hawaiian Archipelago reported here suggest that many of the black coral species found in the Hawaiian Islands have broad distributions across the archipelago. Furthermore, this study is one of the first to examine the microenvironments of sympatric antipatharian species in terms of geographic position, depth and temperature. The results indicate that while there are several similarities in the habitats amongst shallow-water (<150 m) antipatharian species, individual species exploit unique environments in terms of depth or temperature, or possess unique morphologies to minimize overlap.
ACKNOWLEDGEMENTS
I thank D. Opresko for taxonomic assistance, and C. Kelley and J. DeMello for their valuable help during this study. Special thanks to R. Toonen, C. Kelley, J. Drazen, L. Watling, M. Merrifield and three anonymous reviewers for providing insightful reviews and comments on earlier versions of this manuscript. Support in the field was provided by R. Kosaki, J. Leonard, J. Hansen, B. Hauk, K. Gleason, C. Kane, G. McFall, R. Boland, T. Montgomery, P. Murakawa, K. Longenecker, F. Parrish, J. Rooney, Y. Papastamatiou, R. Pyle, R. Whitton, L. Marsh, S. Kahng, J. Eble, K. Ryan, K. Lopes, S. Anendale, J. Heacock and S. Reed. This work was funded in part by the Western Pacific Regional Fisheries Management Council (NA07NMF4410114 to the University of Hawai‘i through NOAA's Coral Reef Conservation Grant Program) and by NOAA's Office of National Marine Sanctuaries, through the Papahānaumokuākea Marine National Monument. The scientific results and conclusions, as well as any views or opinions expressed herein, are those of the authors and do not necessarily reflect the views of NOAA or the Department of Commerce.




