Introduction
The deep-sea shrimp family Glyphocrangonidae Smith, Reference Smith1882, is characterized by the peculiar interlocking articulations of the pleon and telson and seven pairs of longitudinal carinae on the carapace (Holthuis, Reference Holthuis1971; Rice, Reference Rice1981; Chace, Reference Chace1984). Species of the family are widely distributed in the Pacific, Indian and Atlantic Oceans but only found in the deep sea at depths greater than 150 m (e.g. Holthuis, Reference Holthuis1971; Komai, Reference Komai2004a, Reference Komai, Marchall and Richer de Forges2004b, Reference Komai, de Forges and Justine2006; Alves-Júnior et al., Reference Alves-Júnior, de Araújo and Souza-Filho2017; Komai et al., Reference Komai, Chang and Chan2023). Recent studies on this family, particularly in the Indo-West Pacific region, have led to a significant increase in the number of species (Komai, Reference Komai, Marchall and Richer de Forges2004b, Reference Komai2005, Reference Komai, de Forges and Justine2006, Reference Komai2007, Reference Komai2010, Reference Komai2011b; Komai and Chan, Reference Komai and Chan2008, Reference Komai, Chan, Ahyong, Chan, Corbari and Ng2013; Hendrickx, Reference Hendrickx2010; Han and Li, Reference Han and Li2014; Komai et al., Reference Komai, Yang and Chan2020, Reference Komai, Chang and Chan2022), with around 100 currently recognized (WoRMS, 2023). Komai (Reference Komai, Marchall and Richer de Forges2004b) assessed the status of Plastocrangon Alcock, Reference Alcock1901, which was originally established as a subgenus of Glyphocrangon, and concluded that the subgeneric division was not warranted. Komai (Reference Komai, Marchall and Richer de Forges2004b) proposed, instead, the recognition of the two informal species groups within Glyphocrangon, i.e. ‘Glyphocrangon spinicauda A Milne-Edwards, Reference Milne-Edwards1881’ group and ‘G. caeca Wood-Mason in Wood-Mason & Alcock, 1891’ group based mainly on the difference in the gill formula. He also noted, however, the possibility that the G. spinicauda group may not be monophyletic (Komai, Reference Komai, Marchall and Richer de Forges2004b: 387). In this study, we treat species referred to the G. spinicauda species group.
In the sea off Taiwan and the Pratas Island (i.e. Dongsha), northern South China Sea, three (G. formosana Komai, Reference Komai, Marchall and Richer de Forges2004b, G. latens Komai et al., Reference Komai, Chang and Chan2023 and G. perplexa Komai, Reference Komai, Marchall and Richer de Forges2004b) and three species (G. obtusis Komai et al., Reference Komai, Chang and Chan2022, G. stenolepis Chace, Reference Chace1984 and G. unguiculata Wood-Mason in Wood-Mason & Alcock, Reference Wood-Mason and Alcock1891a) of the G. spinicauda group have been confirmed to occur, respectively (Komai et al., Reference Komai, Chan and Lee1998, Reference Komai, Chang and Chan2022, Reference Komai, Chang and Chan2023; Komai, Reference Komai, Marchall and Richer de Forges2004b). The occurrence of other species (i.e. Glyphocrangon granulosis Spence Bate, Reference Spence Bate and Murray1888, G. hastacauda Spence Bate, Reference Spence Bate and Murray1888, G. juxtaculeata Chace, Reference Chace1984, G. pugnax de Man, Reference De Man1918 and G. regalis Bate, 1888) reported by Dong et al. (Reference Dong, Chen, Li, Wang and Wang1988), Zhong (Reference Zhong1989), Han and Li (Reference Han and Li2007) and Liu (Reference Liu2008) in waters around Taiwan and Dongsha has yet to be determined (see Komai, Reference Komai, Marchall and Richer de Forges2004b; Komai et al., Reference Komai, Chang and Chan2022).
Using material collected by Taiwan deep-sea cruises, this study reports five species of Glyphocrangon new to Taiwan and Dongsha, including four new records for Taiwan (G. albatrossae Komai, Reference Komai, Marchall and Richer de Forges2004b, G. caecescens Wood-Mason in Wood-Mason & Alcock, Reference Wood-Mason and Alcock1891b, G. major Komai, Reference Komai, Marchall and Richer de Forges2004b and G. proxima Komai, Reference Komai, Marchall and Richer de Forges2004b) and three new records for Dongsha (G. albatrossae, G. indonesiensis Komai, Reference Komai, Marchall and Richer de Forges2004b and G. proxima). The morphological characters used to distinguish Glyphocrangon grandis Komai & Chan, Reference Komai and Chan2008 from G. major (Komai and Chan, Reference Komai and Chan2008) are found to be unreliable, suggesting that the former taxon is a synonym of G. major. The habitus of each species is illustrated by the micro-computed tomography (micro-CT), a 3D imaging technique utilizing X-rays to see an object slice by slice. This technique has recently gained popularity in crustacean research (e.g. Landschoff et al., Reference Landschoff, Komai, du Plessis, Gouws and Griffiths2018), and is also useful for displaying complex ornamentation on the integumental surface of glyphocrangonid species. Other parts providing diagnostic significance (e.g. ventral surface of the rostrum, antennal scaphocerite and dactyli of fourth and fifth pereopods) are also shown in line drawings. The discovery in the present study brings the total number of species in the G. spinicauda group reported in the waters around Taiwan and Dongsha to 11. A key is provided to aid in the identification of these species, along with information on their biogeographical distributions.
Materials and methods
The specimens used in this study were collected and deposited at the National Taiwan Ocean University in Keelung, Taiwan (NTOU). The measurement of carapace length (cl) (dorsally from the level of the orbital margin to the posterior margin of the carapace) and the morphological terminology followed Komai (Reference Komai, Marchall and Richer de Forges2004b). The station (stn) designations are preceded by a prefix indicating the type of collecting equipment used, as follows: 2.5 m French beam trawl (PCP), 4 m French beam trawl (CP), 3 m ORE beam trawl (OCP) and Le Drezen-type solo hard bottom 12.4 m otter trawl (CD). Other prefixes not mentioned above represent Taiwanese cruises.
Komai (Reference Komai, Marchall and Richer de Forges2004b) showed that species of Glyphocrangon usually exhibit notable sexual differences in the body sculpture (generally better developed in females than in males), the developmental degree of the pleural ventral teeth on the pleon (more acute or strongly produced in females than in males) and the shape of the antennular flagella (stouter in males than in females), in addition to the structure of the first pleopodal endopod and the development of the appendix masculina in males, which are normally seen in Caridea. The characters are briefly mentioned, when necessary. In this study, nevertheless, we highlight the sexual difference in the shape of the penultimate article of the antennular peduncle, which is proportionally stouter in males than in females, like flagella. The proportions of this part show interspecific differences when specimens of the same sex and same ontogenetic stage are compared. When a good series of specimens, covering juvenile and adults, was available, characters showing ontogenetic variation is briefly described.
Specimens for micro-CT scan were preserved in a plastic storage can with 95% alcohol and sent to the Taiwan Mouse Clinic (TMC) for micro-CT scanning. The samples were placed on the scanning bed in a carbon fibre half-tube bed of the Skyscan 1276 and scanned using a desktop micro-CT scanner (SkyScan-1276; Bruker MicroCT N.V., Kontich, Belgium). The scan was conducted at a voxel size of 41.1 μm, with a tube voltage of 70–85 kV and a current of 200 μA. The Al filter used was 0.5–1.0 mm, and the scan was performed at a rotation step of 0.8° and 180° with frame averaging of 2. The micro-CT projections were back reconstructed using NRecon software (Bruker MicroCT) and visualized in 3D with CTVox software (Bruker MicroCT).
Results and discussions
Glyphocrangon albatrossae Komai, Reference Komai, Marchall and Richer de Forges2004b
(Figures 1, 2, 3, 6A)
Glyphocrangon megalophthalma Chace, Reference Chace1984: 18 (in part) [not Glyphocrangon megalophthalma de Man, Reference De Man1918].
Glyphocrangon albatrossae Komai, Reference Komai, Marchall and Richer de Forges2004b: 468, figures 40, 41, 117 (type locality: the Philippines).
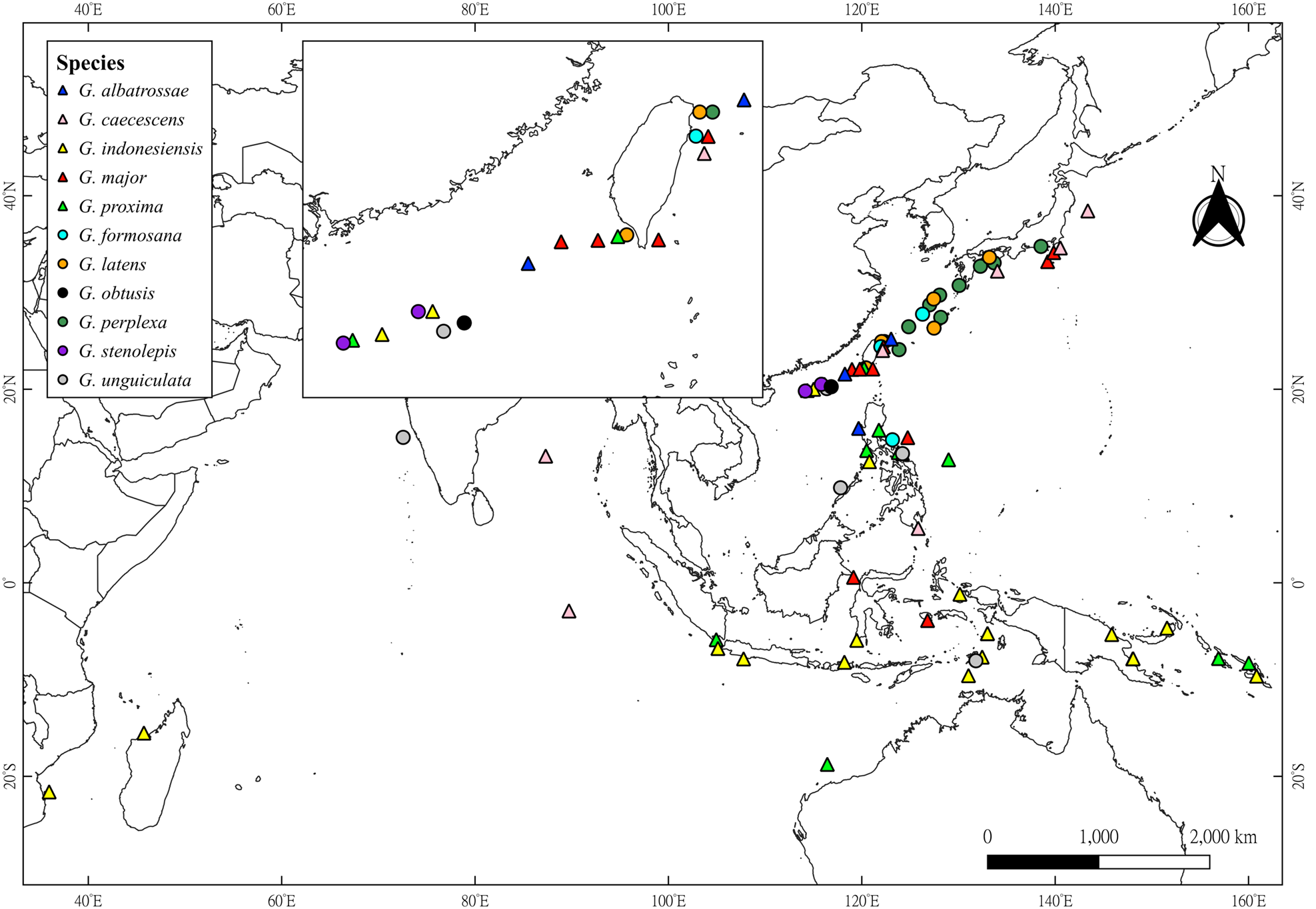
Figure 1. Geographical distributions of the 11 species of Glyphocrangon A. Milne-Edwards, 1881 known in Taiwan and Dongsha. Triangles represent newly recorded species by the present study, while circles denote species that were already known to occur in this sea area. Map was generated using QGIS 3.30.0 (https://qgis.org/en/site/).

Figure 2. Micro-CT images showing general habitus of Glyphocrangon albatrossae Komai, Reference Komai, Marchall and Richer de Forges2004b: (A) ovigerous female cl 23.6 mm (NTOU M02497); (B) male cl 15.9 mm (NTOU M02498). Scale bar: 5 mm.

Figure 3. Glyphocrangon albatrossae Komai, Reference Komai, Marchall and Richer de Forges2004b. (A–D) Ovigerous female cl 23.6 mm (NTOU M02497); (E, F) male cl 15.9 mm (NTOU M02498): (A) ventral surface of distal part of rostrum; (B) left antennal scaphocerite, setae omitted; (C) right fourth pereopod, lateral view; (D) same, dactylus, dorsal view; (E) left antennal scaphocerite, setae omitted; (F) left fourth pereopod, lateral view. Scale bars: A–D, F, 2 mm; E, 3 mm.
Material examined
Taiwan: stn CD238, 25°12.28′N, 123°1.85′E, 1689–1560 m, 23 July 2004, one ovigerous female cl 23.6 mm (NTOU M02497). Dongsha: stn CP190, 21°35.01′N, 118°15.02′E, 1650–1665 m, 28 August 2002, one male cl 15.9 mm (NTOU M02498).
Diagnosis
Female – Body integument with sparse very short setae. Rostrum (Figures 2A, 3A) with two pairs of lateral teeth; dorsal surface with trace of transverse septa. Carapace (Figure 2A) with anterior first (submedian) carina composed of forwardly directed, acute or subacute tubercles; posterior second (intermediate) carina distinctly dentate; anterior third (antennal) carina confined to antennal spine; anterior fourth (lateral) carina entire or faintly uneven on lateral margin; upper part of branchial region with more than 20 (about 30 in present specimen) tubercles; antennal spines strong, diverging anteriorly in dorsal view, ascending in lateral view; branchiostegal spines directed forward, subequal or slightly longer than antennal spine. Second to fourth pleomeres (Figure 2A) each bearing several small to large, blunt tubercles on terga; mid-dorsal carinae moderately high, each deeply divided into two sections. Corneal width 0.20–0.22 times of carapace length. Antennular peduncle penultimate article 2.7–2.8 times as long as distal width. Antennal scaphocerite (Figure 3B) 1.9–2.0 times longer than wide, with lateral tooth arising at 0.4 of its length. Lateral surface of palm of first pereopod glabrous. Dactylus of fourth and fifth pereopods (Figure 3C, D) about 0.4–0.5 times propodus length, subspatulate, each terminating in simple, acuminate unguis, without median carina on flexor surfaces.
Male – Generally similar to female (Figures 2B, 3E, F). Intercarinal spaces on carapace less tuberculate (Figure 2B). Antennular peduncle penultimate article 2.5 times as long as distal width. Lateral tooth of antennal scaphocerite (Figure 3E) more conspicuous than in female.
Colour in life
Body generally ivory white to pale orange. Rostrum, appendages and tail fan reddish to orange; cornea light brown, reflective (Figure 6A).
Distribution
Previously known from the South China Sea, west of Luzon, the Philippines, at depth of 1719 m (Komai, Reference Komai, Marchall and Richer de Forges2004b). The present record extends slightly the distribution of this species northwardly to Dongsha and Taiwan (Figure 1), with vertical distribution of 1560–1719 m deep.
Remarks
G. albatrossae was originally described based on three ovigerous female and one male specimen from the South China Sea, west of Luzon, the Philippines, at a depth of 1719 m (Komai, Reference Komai, Marchall and Richer de Forges2004b), which were previously identified as G. megalophthalma de Man, Reference De Man1918 by Chace (Reference Chace1984). Since the original description, there has been no other record of G. albatrossae.
The morphological characteristics of the present Taiwanese female specimen match the original description of G. albatrossae by Komai (Reference Komai, Marchall and Richer de Forges2004b) (see above ‘Diagnosis’). This species is similar to Glyphocrangon fimbriata Komai & Takeuchi, Reference Komai and Takeuchi1994, G. megalophthalma de Man, Reference De Man1918 and G. richeri Komai, Reference Komai, Marchall and Richer de Forges2004b, but the presence of a trace of transverse septa on the dorsal surface of the rostrum, as also observed in the present specimens (Figure 2), will readily distinguish G. albatrossae from the latter three species (Komai, Reference Komai, Marchall and Richer de Forges2004b).
Like the male specimen described by Komai (Reference Komai, Marchall and Richer de Forges2004b), the present male specimen (cl 15.9 mm, NTOU M02498) differs from the female specimens in the less tuberculate carapace (Figure 2B), but the difference may be due to ontogenetic variation or sexual difference of the same species, which is also seen in the species of Glyphocrangon (Komai, Reference Komai, Marchall and Richer de Forges2004b). Other subtle difference found in the present male specimen is a rather distinct lateral tooth on the antennal scaphocerite, which is obsolescent in the female specimens (Figure 3E vs Figure 3B and figure 41c in Komai, Reference Komai, Marchall and Richer de Forges2004b).
Glyphocrangon caecescens Wood-Mason in Wood-Mason & Alcock, Reference Wood-Mason and Alcock1891b
(Figures 1, 4, 5, 6B)
Glyphocrangon rimapes Spence Bate, Reference Spence Bate and Murray1888: 523 (in part); Rice, Reference Rice1981: 276 (in part), figure D; Brand & Takeda, Reference Brand and Takeda1996: 272, figure 6C, D [not Glyphocrangon rimapes Spence Bate, Reference Spence Bate and Murray1888].
Glyphocrangon caecescens Wood-Mason in Wood-Mason & Alcock, Reference Wood-Mason and Alcock1891b: 357 (type locality: Bay of Bengal); Wood-Mason & Alcock, Reference Wood-Mason and Alcock1894: pl. 7, figure 1; Chace, Reference Chace1984: 5 (key); Komai, Reference Komai, Marchall and Richer de Forges2004b: 450, figures 32, 33, 116; Liu, Reference Liu2008: 724 (list); Komai & Komatsu, Reference Komai and Komatsu2016: 43, figure 8B.
Glyphocrangon sculpta var. coecescens Anonymous, 1891: 54; Huys et al., Reference Huys, Low, De Grave, Ng and Clark2014: 20.

Figure 4. Micro-CT images showing general habitus of Glyphocrangon caecescens Wood-Mason in Wood-Mason & Alcock, Reference Wood-Mason and Alcock1891b, male cl 17.1 mm (NTOU M02499). Scale bar: 5 mm.

Figure 5. Glyphocrangon caecescens Wood-Mason in Wood-Mason & Alcock, Reference Wood-Mason and Alcock1891b, male cl 17.1 mm (NTOU M02499): (A) ventral surface of distal part of rostrum; (B) right antennal scaphocerite, setae omitted; (C) right fourth pereopod, lateral view; (D) same, dactylus, dorsal view. Scale bars: A, B, D, 1 mm; C, 2 mm.

Figure 6. Colouration of fresh specimens: (A) Glyphocrangon albatrossae Komai, Reference Komai, Marchall and Richer de Forges2004b, ovigerous female cl 23.6 mm (NTOU M02497); (B) Glyphocrangon caecescens Wood-Mason in Wood-Mason & Alcock, Reference Wood-Mason and Alcock1891b, male cl 17.1 mm (NTOU M02499); (C) G. indonesiensis Komai, Reference Komai, Marchall and Richer de Forges2004b, ovigerous female cl 24.5 mm (NTOU M02579); (D) G. major Komai, Reference Komai, Marchall and Richer de Forges2004b, ovigerous female cl 34.7 mm (NTOU M02501); (E) G. proxima Komai, Reference Komai, Marchall and Richer de Forges2004b, ovigerous female cl 17.3 mm (NTOU M02531).
Material examined
Taiwan: stn CP4218, 24°01.147′N, 122°09.007′E, 2918–3066 m, 19 January 2021, one male cl 17.1 mm (NTOU M02499).
Diagnosis
Male – Body integument glabrous. Rostrum (Figures 4, 5A) armed with three pairs of lateral teeth; dorsal surface irregularly rugose with trace of incomplete transverse septa. Carapace (Figure 4) with intercarinal spaces covered with numerous small, blunt to acute tubercles; anterior third (antennal) carina confined to antennal spine; anterior fourth (lateral) carina clearly divided in two lobes; antennal spines very strong, diverging anteriorly in dorsal view, ascending in lateral view; branchiostegal spines also diverging in dorsal view, extending beyond antennal spine. Pleon (Figure 4) armed with numerous small conical or subconical tubercles; median carinae on first to fourth somites moderately high. Corneal width 0.15–0.17 times of carapace length. Antennular peduncle penultimate article 2.4 times longer than distal width. Antennal scaphocerite (Figure 5B) 1.8 times longer than wide, with trace of lateral tooth locating at 0.4 of its length. Lateral surface of palm of first pereopod glabrous. Dactyli of fourth and fifth pereopods (Figure 5C, D) 0.3 time longer than propodus, each produced dorsolaterally in acuminate unguis.
Colour in life
Body generally pale orange, with antennae and third maxilliped reddish; cornea light brown, reflective; telson whitish; posterior half of uropodal exopods and posterior one-third length of uropodal endopods transparent (Figure 6B).
Distribution
Widely distributed in the Indo-West Pacific, including Bay of Bengal, Mid Indian Basin, Japan, the Philippines (Komai, Reference Komai, Marchall and Richer de Forges2004b; Komai and Komatsu, Reference Komai and Komatsu2016) and now eastern Taiwan (Figure 1), at depths of 2765–3431 m.
Remarks
G. caecescens was originally described on the basis of a single male specimen from the Bay of Bengal at a depth of 3197 m. There have been no other records by this name until Komai (Reference Komai, Marchall and Richer de Forges2004b) redescribed the species on the basis of material from Mid-Indian Basin, Philippines and Japan, including one of the paratypes of G. rimapes. Recently, Komai and Komatsu (Reference Komai and Komatsu2016) reported the species from off Tohoku District, northern Japan. Among the Indo-West Pacific congenerics, G. caecescens is readily recognized by the possession of three pairs of lateral teeth on the rostrum, numerous conical intercarinal tubercles on the carapace and pleon, the anterior fourth carina on the carapace being primarily divided into two parts by a distinct notch, and terminally bifid dactyli of the fourth and fifth pereopods in adult females (Komai, Reference Komai, Marchall and Richer de Forges2004b; Komai and Komatsu, Reference Komai and Komatsu2016).
Komai (Reference Komai, Marchall and Richer de Forges2004b) briefly described a male specimen of G. caecescens, with which the present male specimen agrees generally. Additional details are: major carinae on carapace (Figure 4) generally composed of denticles or blunt tubercles; anterior fourth (lateral) carina divided into four lobes, the anteriormost lobe forming a blunt tooth anteriorly, second lobe clearly separated from anteriormost lobe, obsolescent at anterior end, posterior two lobes low, obtuse: mid-dorsal carina on pleomeres (Figure 4) each divided into two sections by deep notch, each section on first to fifth pleomeres generally entire or slightly sinuous on dorsal margin, terminating in blunt tooth or becoming obsolescent; mid-dorsal carina on sixth pleomere smooth on dorsal margin, anterior section bilobed, posterior section entire; dorsolateral carinae (Figure 4) on first pleomere high, strongly compressed laterally, showing as triangular tooth with subacute apex; dorsolateral carinae on second to fourth pleomeres generally composed by conical tubercles or greatly reduced. Telson (Figure 4) dorsolateral carinae tuberculate in anterior 0.7, ventrolateral carina tuberculate in anterior 0.2.
The living colour of the present Taiwan specimen is rather similar to the description by Wood-Mason and Alcock (Reference Wood-Mason and Alcock1891b) that stated ‘pale pink, corneae dull yellow’. The specimens from off Tohoku District, northern Japan, in contrast, are generally red with brown corneas; corneas in preservative are darkly pigmented (Komai and Komatsu, Reference Komai and Komatsu2016). Molecular comparison is recommended to establish the identity of the specimens reported by Komai and Komatsu (Reference Komai and Komatsu2016).
Glyphocrangon indonesiensis Komai, Reference Komai, Marchall and Richer de Forges2004b
(Figures 1, 6C, 7, 8)
Glyphocrangon hastacauda de Man, Reference De Man1920: 224; Chace, Reference Chace1984: 13; Takeda & Hanamura, Reference Takeda and Hanamura1994: 28 [not Glyphocrangon hastacauda Spence Bate, Reference Spence Bate and Murray1888].

Figure 7. Micro-CT images showing general habitus of Glyphocrangon indonesiensis Komai, Reference Komai, Marchall and Richer de Forges2004b: (A) female cl 22.1 mm (NTOU M02506); (B) male cl 21.8 mm (NTOU M02505). Scale bar: 5 mm.

Figure 8. Glyphocrangon indonesiensis Komai, Reference Komai, Marchall and Richer de Forges2004b. (A–D) Female cl 22.1 mm (NTOU M02506); (E, F) male cl 21.8 mm (NTOU M02505): (A) ventral surface of distal part of rostrum; (B) left antennal scaphocerite, setae omitted; (C) left fourth pereopod, lateral view; (D) same, dactylus, dorsal view; (E) left antennal scaphocerite, setae omitted. Scale bars: A, D, 2 mm; B, E, 4 mm; C, 3 mm.
Glyphocrangon indonesiensis Komai, Reference Komai, Marchall and Richer de Forges2004b: 408, figures 10, 11, 114; 2011b: 122 (type locality: Tanimbar Island, Banda Sea, Indonesia); Komai & Chan, Reference Komai, Chan, Ahyong, Chan, Corbari and Ng2013: 115, figures 2C, 4; Komai et al., Reference Komai, Yang and Chan2020: 638, figure 1B.
Material examined
Dongsha: stn CP4118, 20°00.7563′N, 115°00.8310′E, 700–723 m, 12 January 2014, four females cl 7.4–17.9 mm, two males cl 15.2 mm, 11.1 mm (NTOU M02503); stn CP4119, 12 January 2014, 20°01.3340′N, 115°00.1120′E, 653–723 m, one female cl 12.9 mm (NTOU M02504); stn CP4129, 20°31.375′N, 116°08.002′E, 590–633 m, 2 May 2014, one female cl 22.1 mm (NTOU M02506); same station, one male cl 21.8 mm (NTOU M02505); stn CP4130, 20°17.971′N, 116°07.966′E, 795–822 m, 2 May 2014, one female cl 11.0 mm (NTOU M02507); 580 m, 25 March 2022, five ovigerous females cl 24.2–26.8 mm (NTOU M02578); same data, one ovigerous female cl 24.5 mm (NTOU M02579).
Diagnosis
Female – Body integument covered with dense pubescence. Rostrum (Figures 7A, 8A) with two pairs of lateral teeth; dorsal surface without transverse septa or corrugation; ventral surface tricarinate in about anterior 1/3. Carapace (Figure 7A) without conspicuous tubercles on intercarinal spaces; first (submedian) carina nearly entire; anterior third (antennal) carina nearly entire, extending to upper part of hepatic region; anterior fourth (lateral) carina entire, not expanded, posterior fourth (lateral) carina unarmed at anterior end; antennal spine strong, directed forward in dorsal view, weakly ascending in lateral view; branchiostegal spines short, not reaching antennal spines. First to fifth pleomeres (Figure 7A) with short ridges or small tubercles on tergum; second and third pleomeres with mid-dorsal carinae low, not crested, posterior section on third pleomere not produced posterodorsally; mid-dorsal carina on fourth pleomere entire or faintly divided by shallow notch; lateral carina on sixth pleomere entire. Corneal width 0.24–0.27 times of carapace length. Antennular peduncle penultimate article 2.0–2.5 times as long as distal width. Antennal scaphocerite (Figure 8B) 1.6–1.9 times longer than wide. Lateral surface of palm of first pereopod glabrous. Dactyli of fourth and fifth pereopods (Figure 8C, D) 0.6–0.7 and 0.4–0.5 times longer than propodus, respectively, each subspatulate, terminating in simple, acuminate unguis.
Male – Generally similar to female (Figure 7B). Terga of pleomeres less sculptured, median carina lower. Antennular peduncle penultimate article 1.7–2.2 times longer than distal width. Antennal scaphocerite (Figure 8E) 1.5–1.8 times longer than wide.
Ontogenetic variation
The smaller specimens (cl <15 mm) in the present series have somewhat crested mid-dorsal carinae on the second to third pleomeres and a distinctly divided mid-dorsal carina on the fourth somite, but their mid-dorsal carina on the second pleomere is not produced anterodorsally with an acute tooth as in G. hastacauda (Komai, Reference Komai, Marchall and Richer de Forges2004b: figure 8). The penultimate article of the antennular peduncle (2.7–3.2 times of distal width) and the antennal scaphocerite (mostly 1.8–2.4 times longer than width) are also slenderer than those in adult specimens (see above ‘Diagnosis’).
Colour in life
Carapace and rostrum generally whitish, with first (submedian) and second (intermediate) carina and edge of rostrum reddish; pleomeres orange dorsally and whitish laterally; tail fan generally reddish; pereopods generally whitish, with dactyli and distal half of propodus orange to red (Figure 6C).
Distribution
Widely distributed in the Indo-West Pacific, including Madagascar, Indonesia, Papua New Guinea, Solomon Islands, the Philippines (Komai, Reference Komai, Marchall and Richer de Forges2004b, Reference Komai2011b; Komai and Chan, Reference Komai, Chan, Ahyong, Chan, Corbari and Ng2013; Komai et al., Reference Komai, Yang and Chan2020) and now off Dongsha (Figure 1), at a depth of 200–1150 m (usually 600–900 m).
Remarks
Takeda and Hanamura (Reference Takeda and Hanamura1994) noticed morphological differences between specimens referred to G. hastacauda Spence Bate, Reference Spence Bate and Murray1888 from Japan and Indonesia. Komai (Reference Komai, Marchall and Richer de Forges2004b) later examined a large number of specimens from Japan, Indonesia and the Philippines, and concluded that the Indonesian and Philippine material represented a separate species, described as a new species G. indonesiensis.
The present study includes 16 specimens of G. indonesiensis, ten of which are full adults (cl >15 mm). These adult specimens match G. indonesiensis in every diagnostic aspect, such as the low, non-crested mid-dorsal carina on the second to third pleomeres, the mid-dorsal carina on the fourth pleomere lacking a deep notch, and the short ridges or small tubercles present on the tergum of the first to fifth pleomeres (see Figure 7 and figure 10 in Komai, Reference Komai, Marchall and Richer de Forges2004b). Glyphocrangon hastacauda, on the other hand, has crested, well-produced median carinae on the second and third pleomeres (Komai, Reference Komai, Marchall and Richer de Forges2004b: figure 8). It is interesting to note that no specimens of G. hastacauda have been collected from waters around Taiwan, although the species extends to the northern part of the East China Sea (Komai, Reference Komai, Marchall and Richer de Forges2004b). The case of G. indonesiensis and G. hasacauda pair may represent an example of vicariant speciation.
Glyphocrangon major Komai, Reference Komai, Marchall and Richer de Forges2004b
(Figures 1, 6D, 9, 10)
Glyphocrangon granulosis de Man, Reference De Man1920: 230, pl. 19, figure 58a–f; Chace, Reference Chace1984: 12 (in part) [not Glyphocrangon granulosis Spence Bate, Reference Spence Bate and Murray1888].
Glyphocrangon cf. granulosis Watabe & Miyake, Reference Watabe and Miyake2000: 32.
Glyphocrangon major Komai, Reference Komai, Marchall and Richer de Forges2004b: 514, figures 63, 64, 118 (type locality: Makassar Strait and south of Buru, Indonesia); 2011a: 289, figure 4.
Glyphocrangon grandis Komai & Chan, Reference Komai and Chan2008: 41, figures 1–5, 12D (type locality: Lagonoy Gulf, Philippines, 1037–1100 m).

Figure 9. Micro-CT images showing general habitus of Glyphocrangon major Komai, Reference Komai, Marchall and Richer de Forges2004b: (A) ovigerous female cl 34.7 mm (NTOU M02501); (B) female cl 18.0 mm (NTOU M02518); (C) male cl 29.3 mm (NTOU M02522). Scale bar: 5 mm.

Figure 10. Glyphocrangon major Komai, Reference Komai, Marchall and Richer de Forges2004b. (A–D) Ovigerous female cl 34.7 mm (NTOU M02501); (E, F) male cl 29.3 mm (NTOU M02522): (A) ventral surface of distal part of rostrum; (B) left scaphocerite, setae omitted; (C) right fourth pereopod, lateral view; (D) same, dactylus, dorsal view; (E) left scaphocerite, setae omitted; (F) right fourth pereopod, lateral view. Scale bars: A, 3 mm; B, C, 6 mm, D–F, 2 mm.
Material examined
Taiwan: stn CD199, 24°25.38′N, 122°12.41′E, 1138–1187 m, 12 September 2002, one ovigerous female cl 42.4 mm, one female cl 42.8 mm, one male cl 33.8 mm, three juveniles cl 11.7–15.6 mm (NTOU M 02580); stn CD228, 22°8.7′N, 121°0.97′E, 1259–1383 m, 30 August 2003, one female cl 12.2 mm, one male cl 17.8 mm (NTOU M02508); stn CP277, 24°23.57′N, 122°14.12′E, 1222–1261 m, 14 June 2005, one male cl 14.7 mm (NTOU M02509); same station, one male cl 27.5 mm (NTOU M02510); stn OCP280, 24°23.71′N, 122°14.22′E, 1213–1261 m, 14 June 2005, one male cl 17.8 mm (NTOU M02511); stn CP281, 24°24.08′N, 122°14.06′E, 1173–1248 m, 15 June 2005, one ovigerous female cl 35.3 mm (NTOU M02500), one female cl 14.1 mm (NTOU M02512), one male cl 10.6 mm (NTOU M02513); stn OCP282, 24°23.90′N, 122°14.10′E, 1200–1250 m, 15 June 2005, one female cl 21.3 mm (NTOU M02514); stn CP363, 22°09.305′N, 121°07.353′E, 1262–1269 m, 24 August 2006, one female cl 7.6 mm, one male cl 10.6 mm (NTOU M02515); stn CP364, 22°06.335′N, 121°08.224′E, 1275–1260 m, 24 August 2006, one male cl 17.6 mm (NTOU M02516); stn CP372, 24°23.619′N, 122°14.138′E, 1220–1280 m, 26 August 2006, one male cl 11.7 mm (NTOU M02517); stn PCP458, 22°16.760′N, 121°3.110′E, 1193–1229 m, 26 July 2010, one male cl 27.0 mm (NTOU M 02581); stn CP4167, 22°06.13′N, 119°07.78′E, 1756–1306 m, 1 August 2015, one ovigerous female cl 34.7 mm (NTOU M02501), one female cl 18.0 mm (NTOU M02518); same station, two females cl 12.2 mm, 12.6 mm (NTOU M02519); same station, one female cl 34.1 mm (NTOU M02502); Cold Seep Cruise 2016, stn CST8, 22°5.728′N, 119°47.695′E, 1669–1458 m, 26 April 2016, one female cl 33.8 mm (NTOU M02520); same project, stn CST17, 22°3.791′N, 118°58.804′E, 1483 m, 1 May 2016, one male cl 15.2 mm (NTOU M02521); Cold Seep Cruise 2018, stn S-GWR2-2, 22°10.859′N, 119°52.913′E, 1300–1375 m, 11 April 2018, one male cl 29.3 mm (NTOU M02522); same project, stn FWC-G2, 22°00.149′N, 119°48.051′E, 1660–1340 m, 10 April 2018, one female cl 17.6 mm (NTOU M02523); same project, one female cl 31.1 mm, one male carapace broken (NTOU M02524).
Diagnosis
Female – Body integument glabrous. Rostrum (Figures 9A, 10A) with two pairs of lateral teeth; dorsal surface without sculpture; median carina absent on ventral surface. Carapace (Figure 9A) covered with high, strongly compressed tubercles; anterior first (submedian) carina composed of high, compressed tubercles with blunt to subacute apices; anterior third (antennal) carina confined to antennal spine, posterior third (antennal) carina at most angular at anterior end; anterior fourth (lateral) carina distinctly divided into two sections; antennal spine strong, diverging in dorsal view, ascending in lateral view; branchiostegal spines directed forward, overreaching antennal spines. Pleon covered with numerous prominent tubercles, most tubercles conical or subconical, terminating in blunt apices; second to fourth pleomeres (Figure 9A) with mid-dorsal carinae highly crested, each divided into two sections by deep notch. Corneal width 0.17–0.20 time of carapace length. Antennular peduncle penultimate article 1.9–2.6 times longer than distal width. Antennal scaphocerite (Figure 10B) 1.6–2.0 times longer than wide, with lateral spine arising from anterior to or mid-length of lateral margin. Lateral surface of palm of first pereopod glabrous. Dactylus of fourth and fifth pereopods (Figure 10C, D) 0.3–0.4 times longer than propodus, each subspatulate, terminating in simple, acuminate unguis, without median carina on flexor surface.
Male – Generally similar to females (Figure 9C, 10F). Antennular peduncle penultimate article 1.9–2.2 times longer than distal width. Antennal scaphocerite (Figure 10E) 1.7 times longer than wide.
Ontogenetic variation
Komai (Reference Komai, Marchall and Richer de Forges2004b) described differences between the holotype and subadult female paratype (cl 23.4 mm). The present material enabled us to examine ontogenetic variation in G. major in more detail, and the differences described by Komai (Reference Komai, Marchall and Richer de Forges2004b) for the paratype are also observed in the present specimens with similar body size (cl 17.6–21.3 mm, NTOU M02508, M02514, M02516, M02518, M02523) (Figure 9B). A faint trace of transverse septa, furthermore, is observed on the dorsal surface of the rostrum (Figure 9B), which is barely visible to the naked eyes. Moreover, the penultimate article of the antennular peduncle (2.3–3.0 times longer than distal width) and antennal scaphocerite (1.8–2.1 times longer than width) are longer in small male specimens (cl <20 mm) than those in larger specimens of the same sex.
Colour in life
Carapace reddish with upper part of hepatic region dark brown; terga of pleon whitish to orange with densely reddish carinae and tubercles, giving it an overall crimson appearance (Figure 6D).
Distribution
Previously known from Indonesia (Komai, Reference Komai, Marchall and Richer de Forges2004b), Japan (Komai, Reference Komai2011a) and the Philippines (Komai and Chan, Reference Komai and Chan2008; see more detail in Remarks), at depths of 1009–1330 m. Now newly recorded from Taiwan, at depths of 1138–1756 m, slightly extending the bathymetric range (Figure 1).
Remarks
Komai (Reference Komai, Marchall and Richer de Forges2004b) examined specimens referred to G. granulosis Spence Bate, Reference Spence Bate and Murray1888, including the holotype and paratype, and concluded that the specimens identified with G. granulosis by de Man (Reference De Man1920) and Chace (Reference Chace1984) represented a separate species named as G. major. Komai and Chan (Reference Komai and Chan2008) subsequently described another species G. grandis based on a single female specimen from the Lagonoy Gulf, the Philippines, in comparison with G. major. Komai (Reference Komai2011a) recorded G. major from Japanese waters for the first time. Watabe and Miyake (Reference Watabe and Miyake2000) provisionally identified a specimen collected from the southern Ryukyu Islands with G. cf. granulosis.
Komai and Chan (Reference Komai and Chan2008) discriminated G. grandis from G. major based on the shape of the anterior end of the posterior fourth (lateral) carina on the carapace (acutely pointed, spine-like in G. grandis vs blunt in G. major) and of the anterior vertical ridges on the pleura of the third and fourth pleomeres (terminating ventrally in spine in G. grandis vs in tubercle in G. major). Examination of the present series of specimens, however, revealed that those characters are variable. For example, one ovigerous female specimen (cl 35.3 mm, NTOU M02500) has a blunt anterior end on the left side and a sharp, spiniform anterior end on the right side of the posterior fourth (lateral) carina, but sharp spines on the anterior vertical ridges on the third and fourth pleonal pleura. The other two female specimens (cl 31.1 mm, NTOU M02524; cl 21.3 mm, NTOU M02514) have blunt ends on the anterior vertical ridges on the third and fourth pleonal pleura, but an acute tooth on the anterior end of the posterior fourth (lateral) carina. We thus concluded that the two nominal taxa are conspecific, and synonymize G. grandis under G. major.
Glyphocrangon major is one of the largest species among congeners, attaining 40 mm in the carapace length in the female (Komai, Reference Komai, Marchall and Richer de Forges2004b). Among the male specimens reported in the present study, four had a carapace length more than 20 mm (NTOU M02510, NTOU M02522, NTOU M02580, NTOU M02581), and they (Figure 9C) are morphologically similar to female specimens, suggesting that sexual dimorphism in the body sculpture is not developed in this species.
Glyphocrangon proxima Komai, Reference Komai, Marchall and Richer de Forges2004b
(Figures 1, 6E, 11, 12)
Glyphocrangon gilesii Chace, Reference Chace1984: 11 (in part) [not Glyphocrangon gilesii Wood-Mason in Wood-Mason & Alcock, Reference Wood-Mason and Alcock1891b.
Glyphocrangon proxima Komai, Reference Komai, Marchall and Richer de Forges2004b: 416, figures 14, 15, 115 (type locality: Kai Islands, Banda Sea, Indonesia); 2011b: 131; Komai & Chan, Reference Komai and Chan2008: 52, figure 13C1, C2; 2013: 119; Komai et al., Reference Komai, Yang and Chan2020: 645, figure 1C.

Figure 11. Micro-CT images showing general habitus of Glyphocrangon proxima Komai, Reference Komai, Marchall and Richer de Forges2004b: (A) ovigerous female cl 17.2 mm (NTOU M02532); (B) male cl 14.2 mm (NTOU M02528). Scale bar: 5 mm.

Figure 12. Glyphocrangon proxima Komai, Reference Komai, Marchall and Richer de Forges2004b. (A–D) Ovigerous female cl 17.3 mm (NTOU M02531); (E, F) male cl 15.2 mm (NTOU M02525): (A) ventral surface of distal part of rostrum; (B) left scaphocerite, setae omitted; (C) left fourth pereopod, lateral view; (D) same, dactylus, dorsal view; (E) left scaphocerite, setae omitted; (F) left fourth pereopod, lateral view. Scale bars: 1 mm.
Material examined
Taiwan: stn CD133, 22°15.07′N, 120°8.02′E, 748–690 m, 21 November 2001, two females cl 14.4 mm, 11.6 mm (NTOU M02526); same station, one male cl 15.2 mm (NTOU M02525); stn CD138, 22°13.13′N, 120°20.17′E, 441–789 m, 23 November 2001, one male cl 12.1 mm (NTOU M02527); stn CD139, 22°10.73′N, 120°14.1′E, 852–718 m, 23 November 2001, one male cl 14.2 mm (NTOU M02528); stn PCP 447, 22°12.384′N, 120°14.423′E, 813–819 m, 14 July 2008, one female cl 13.5 mm (NTOU M02529); stn PCP475, 22°04.222′N, 120°16.490′E, 858–727 m, 30 July 2014, one female cl 13.6 mm (NTOU M02530). Dongsha: stn CP4137, 19°53.06′N, 114°21.68′E, 536–524 m, 23 July 2015, one ovigerous female cl 17.3 mm (NTOU M02531); same station, two ovigerous females cl 16.2 mm, 17.2 mm, one female cl 16.9 mm (NTOU M02532).
Diagnosis
Female – Body integument glabrous. Rostrum (Figures 11A, 12A) with two pairs of lateral teeth; dorsal surface without transverse septa. Carapace (Figure 11A) at most with few, indistinct tubercles on intercarinal spaces; first (submedian) carina moderately high, each section terminating anteriorly in blunt point; posterior second (intermediate) carina entire; anterior third (antennal) carina extending to upper part of hepatic region, divided into two parts; posterior third (antennal) carina entire, with blunt end anteriorly; anterior fourth (lateral) carina not divided or expanded, terminating in blunt or obsolescent point anteriorly; antennal spines strong, slightly diverging in dorsal view, gently ascending in lateral view; branchiostegal spines distinctly shorter than antennal spines. Pleon (Figure 11A) with some small, conspicuous tubercles on terga and pleura of first to fourth pleomeres, each mid-dorsal carina low; third and fourth pleura with anterior vertical ridges faded away ventrally. Corneal width 0.22–0.27 times of carapace length. Antennular peduncle penultimate article less than four times (3.1–3.7 times in the present specimens) longer than distal width. Antennal scaphocerite (Figure 12B) elongate oval, 1.9–2.1 times longer than wide, with acute lateral tooth situated posterior to midlength of scaphocerite. Lateral surface of palm of first pereopod glabrous. Dactyli of fourth and fifth pereopods (Figure 12C, D) 0.5–0.7 and 0.4–0.5 times longer than propodus, each subspatulate, terminating in simple, acuminate unguis.
Male – Generally similar to females (Figures 11B, 12F). Antennular peduncle penultimate article 1.9–2.3 times as long as distal width (Figure 12E).
Ontogenetic variation
Antennular peduncle penultimate article more than four times as long as distal width in smallest female specimen (cl 11.6 mm, NTOU M02526).
Colour in life
See Komai and Chan (Reference Komai and Chan2008); antennular peduncle, antennal scaphocerite, pleonal pleura, pleopods and uropods whitish to translucent in present specimens (Figure 6E).
Distribution
Widely distributed in the West Pacific, from the Philippines, northwestern Australia, Indonesia to Solomon Islands (Komai, Reference Komai, Marchall and Richer de Forges2004b, Reference Komai2011b; Komai and Chan, Reference Komai and Chan2008, Reference Komai, Chan, Ahyong, Chan, Corbari and Ng2013; Komai et al., Reference Komai, Yang and Chan2020). The present study extends the distribution of this species northwardly to Dongsha and Taiwan (Figure 1), at depths of 482–980 m.
Remarks
G. proxima was originally described based on material from Indonesia, the Philippines and northwestern Australia, including specimens referred to as G. gilesii Wood-Mason in Wood-Mason & Alcock, Reference Wood-Mason and Alcock1891b by Chace (Reference Chace1984) (Komai, Reference Komai, Marchall and Richer de Forges2004b). As discussed by Komai (Reference Komai, Marchall and Richer de Forges2004b), G. proxima, G. gilesii and G. runcinata Komai, Reference Komai, Marchall and Richer de Forges2004b are morphologically very similar to one another. Diagnostic characters of G. gilesii have remained to be reassessed based on the topotypic material from the Andaman Sea.
Examination of the present specimens from Taiwan and Dongsha has revealed that variation is seen in some characters that have been considered diagnostic to the species. In one ovigerous female specimen (cl 17.2 mm, NTOU M02532) collected off Dongsha, the anterior end of the first (submedian) carina is subacutely pointed, rather than bluntly pointed. The second tooth of the anterior second (intermediate) carina in two of the three ovigerous female specimens collected off Dongsha (cl 16.2 and cl 17.2 mm, NTOU M02532) was acute or subacute; in the female specimen from Taiwan (cl 14.4 mm NTOU M02526), the left side of that carina is acute, although the right side is blunt. Other diagnostic characters for G. proxima proposed by Komai (Reference Komai, Marchall and Richer de Forges2004b) seem to be still useful.
Key to species of ‘G. spinicauda’ species group in the waters around Taiwan and Dongsha
Eleven species belonging to the G. spinicauda species group are now known in the waters around Taiwan and Dongsha (Komai et al., Reference Komai, Chan and Lee1998, Reference Komai, Chang and Chan2022, Reference Komai, Chang and Chan2023; Komai, Reference Komai, Marchall and Richer de Forges2004b; Liu, Reference Liu2008), including five that are newly reported in this study. These are: G. albatrossae, G. caecescens, G. formosana, G. indonesiensis, G. latens, G. major, G. obtusis, G. perplexa, G. proxima, G. stenolepis and G. unguiculata.
The records of other species in this group, such as G. granulosis, G. hastacauda, G. juxtaculeata, G. pugnax and G. regalis, are yet to be confirmed as they are only listed in previous reports (cf. Dong et al., Reference Dong, Chen, Li, Wang and Wang1988; Zhong, Reference Zhong1989; Liu, Reference Liu2008). Moreover, G. granulosis and G. regalis are so far represented only by the holotypes collected in New Guinea and Indonesia, respectively (Komai, Reference Komai, Marchall and Richer de Forges2004b), while G. juxtaculeata is known only with certainty from the type locality in Indonesia and the subsequent record from off Java, Indonesia (Komai et al., Reference Komai, Chang and Chan2022). Although G. hastacauda has been recorded from Japan and the northern part of the East China Sea (Komai, Reference Komai, Marchall and Richer de Forges2004b), the species has not been recorded from waters around Taiwan in spite of recent active deep-sea investigations. Glyphocrangon pugnax has been recorded from the Philippines, NW Australia, Indonesia and the Solomon Islands (Komai, Reference Komai, Marchall and Richer de Forges2004b, Reference Komai2011b; Komai and Chan, Reference Komai and Chan2008, Reference Komai, Chan, Ahyong, Chan, Corbari and Ng2013; Komai et al., Reference Komai, Yang and Chan2020), but no specimens of the species have been collected from waters around Taiwan too.
The key to the local species provided below is based on Komai (Reference Komai, Marchall and Richer de Forges2004b) with some emendation and can be used to identify adult specimens of both sexes of the aforementioned 11 species known with certainty in the waters off Taiwan and Dongsha, with the exception of G. formosana, for which no male specimens have been discovered to date (Komai, Reference Komai, Marchall and Richer de Forges2004b). Characters that are specific to male specimens will be indicated in the key.
1. Anterior third (antennal) carina extending to upper part of hepatic region, occasionally divided into two parts………2
− Anterior third (antennal) carina limited to antennal spine, but a row of small tubercles aligned to antennal spine may be present on upper part of hepatic region………3
2. Anterior third (antennal) carina entire; integument of carapace and pleon covered with dense pubescence……… ………G. indonesiensis
− Anterior third (antennal) carina divided into two parts; integument of carapace and pleon glabrous………G. proxima
3. Rostrum with three pairs of lateral teeth. Dactyli of fourth and fifth pereopods somewhat flattened but not subspatulate, with horizontal cleft distally in females, dorsolaterally terminating in acuminate unguis in male………G. caecescens
− Rostrum with two pairs of lateral teeth. Dactyli of fourth and fifth pereopods subspatulate, terminating in simple, acuminate unguis (except for female G. unguiculata)………4
4. Anterior fourth (lateral) carina divided into two teeth or lobes, but not particularly expanded………5
− Anterior fourth (lateral) carina expanded into wide, undivided, vertically compressed acute lamina………10
5. Body integument covered with dense pubescence; in females, dactyli of fourth and fifth pereopods horizontally cleft distally ………G. unguiculata
− Body integument glabrous or at most with sparse short setae; dactyli of fourth and fifth pereopods terminating in simple acuminate unguis even in females………6
6. Carapace branchiostegal spine twice as long as antennal spine. First (submedian) carina on carapace relatively low, with anterior section composed of 4–6 lobes………7
− Carapace branchiostegal spine less than twice as long as antennal spine. First (submedian) carina relatively high, with anterior section composed of several blunt to acute tubercles or denticles………9
7. Dorsal surface of rostrum with or without trace of transverse septa. Anterior section of anterior fourth (lateral) carinae obtuse at anterior end………G. obtusis
− Dorsal surface of rostrum with distinct transverse septa. Anterior section of anterior fourth (lateral) carinae on carapace terminating in acute or subacute tooth………8
8. First (submedian) carinae on carapace clearer divided. Carina on pleon better developed in females. In males, trace of dorsolateral and mid-dorsal carinae on the second to fourth pleomeres discernible………G. latens
− First (submedian) carinae on the carapace faintly divided. Carina on the pleon obsolescent to absent in females. In males, dorsolateral and mid-dorsal carinae on second to fourth pleomeres absent………G. stenolepis
9. Intercarinal spaces of carapace partially covered with blunt, not strongly compressed tubercles (mainly on dorsal surface and upper part of branchial region). Second to fourth pleomeres with median carina not highly crested, terga and pleura covered with low and broad tubercles………G. albatrossae
− Intercarinal spaces of carapace largely covered with strongly compressed tubercles. Second to fourth pleomeres with median carina highly crested, terga and pleura covered with numerous and long tubercles………G. major
10. Ventral surface of rostrum without median carina; palm of first pereopod glabrous on lateral surface………G. perplexa
− Ventral surface of rostrum with median carina; palm of first pereopod with numerous short setae on lateral surface……… ………G. formosana
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgements
The authors sincerely thank Tin-Yam Chan of National Taiwan Ocean University, Taiwan ROC for providing the material for this study.
Author contributions
S.-C. Chang identified species, edited figures, wrote the first draft, provided funding resources and facilitated communication among all authors involved in this research; C.-L. Chen analysed and produced the micro-CT images, as well as edited figures; J.-Y. Chen identified species, produced line drawing and edited figures; T. Komai reviewed the manuscript, provided critical suggestions and comments on this research. All authors contributed to the refinement of the final manuscript.
Financial support
This work was supported by the research grants from the National Science and Technology Council, Taiwan, ROC.
Competing interest
The authors declare that there are no known competing financial interests or personal relationships that could potentially influence the findings reported in this study.
Ethical standards
This work is not applicable to ethical standards.














