Published online by Cambridge University Press: 21 July 2014
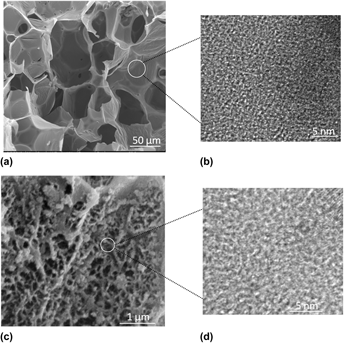
Adsorbents with high specific surface areas, developed porosities, and sustainable CO2 capture capacity (∼180 mg/g at 25 °C, 1 bar) were prepared by KOH activation of hydrothermally carbonized carboxymethylcellulose (CMC). Condensed aromatic carbon materials (CSc) with particle diameters of 2–3 μm and many oxygen-containing groups on their surfaces can be obtained after hydrothermal treatment of CMC; these materials are similar to glucose-derived hydrothermal carbons. The activation conditions, including activation ratio and activation temperature, significantly influence the structure and morphology of the adsorbents. In turn, the pore structures, specific surface areas, and adsorption conditions significantly affect the adsorption capacities of these new adsorbents. For samples with the same activation ratio, those with higher specific surface areas show higher CO2 capture capacities at 25 °C and 1 bar. Under these conditions, for samples with different activation ratios, the capacity is dominated by the microporosity development and, in particular, the high volume of smaller micropores (d = 0.4–0.9 nm); when the adsorption pressure is decreased to 0.1 bar, the CO2 capture ability becomes closely correlated with the number of ultramicropores (d < 0.7 nm).