Introduction
Trichinella spiralis (T. spiralis) is a zoonotic roundworm that causes trichinellosis. Trichinellosis is one of the most significant foodborne parasitic infection worldwide according to WHO (2021). It is considered a public health hazard and a dangerous threat to meat and food safety (Bai et al., Reference Bai, Hu, Liu, Tang and Liu2017). A large number of cases occurs annually by eating raw or undercooked pork or its products containing infective larvae (Basso et al., Reference Basso, Marreros, Hofmann, Salvisberg, Lundström-Stadelmann and Frey2022).
T. spiralis adult worms live in the intestinal mucosa of mammals, birds, and reptiles and the larvae are established in skeletal muscle. Therefore, the course of T. spiralis infection in humans consists of two major phases: intestinal phase, which is characterised by abdominal pain, diarrhea, nausea, and vomiting and the muscular phase, which is caused by the inflammatory and allergic responses induced by muscular invasion and may be accompanied with fever, myalgia, eosinophilia, and eyelid edema (Bruschi, Reference Bruschi and Bruschi2014).
When larvae invade skeletal muscle cells, this leads to major cell modifications (Farid et al., Reference Farid, Fath, Mido, Nonaka and Horii2019), transforming the fully differentiated muscle cells into nurse cells. These cells are consisted of host-derived collagenous capsule that sustain the muscle larvae and cellular components (Xu et al., Reference Xu, Bai, Wang, Shi, Van Der Giessen, Boireau, Liu and Liu2017). This larval invasion can damage muscle cells directly or indirectly by stimulating inflammatory cell infiltration and altering pro-inflammatory cytokines and antioxidant capacity, leading to tissue damage (Gottstein et al., Reference Gottstein, Pozio and Nöckler2009; Soliman et al., Reference Soliman, Shafik, Shoheib and Ashour2013). Furthermore, the oxidative stress that occurs during trichinellosis is considered one of the major causes of tissue damage. Extreme amounts of reactive oxygen species, nitrogen species, and free radicals are produced by the activation of recruited inflammatory cells (Chiumiento & Bruschi, Reference Chiumiento and Bruschi2009). Additionally, elevated levels of many stress indicators, such as malondialdehyde and superoxide dismutase, were detected (Othman et al., Reference Othman, Abou Rayia, Ashour, Saied, Zineldeen and El-Ebiary2016).
The prevalence of trichinellosis is rising worldwide because of treatment difficulties. These difficulties are attributed to limited effectiveness of the specific medications to inhibit migrating and encysted larvae. Benzimidazole derivatives, such as mebendazole and albendazole, are the main antiparasitic drugs used against trichinellosis. However, their action is insufficient against the encapsulated larvae besides increasing resistance to them. Furthermore, they exhibited low water solubility that makes their absorption from the intestinal lumen limited and results in low bioavailability (Khalikov, Reference Khalikov2021). In addition, mebendazole has shown teratogenicity in various experimental animals (de la Torre-Iglesias et al., Reference de la Torre-Iglesias, García-Rodriguez, Torrado, Torrado, Torrado-Santiago and Bolás-Fernández2014). Accordingly, natural medications and potent antioxidants are fundamentally needed (Nada et al., Reference Nada, Mohammad, Moad, EL-Shafey, Al-Ghandour and Ibrahim2018).
Ellagic acid (EA) is a valuable bioflavonoid found in various vegetables, fruits, and berries. EA is a thermostable dilactone of hexahydroxydiphenic acid (Vattem et al., Reference Vattem, Ghaedian and Shetty2005). EA has effective antioxidant activities and important therapeutic benefits on the limitation of many chronic pathological disorders associated with oxidative stress, so it takes huge attention from biomedical researchers (Shakeri et al., Reference Shakeri, Zirak and Sahebkar2018). Additionally, it has numerous health profits such as anti-inflammatory, antibacterial, antiviral, anticancer, hepatoprotective, gastroprotective, and cardio-protective properties (Evtyugin et al., Reference Evtyugin, Magina and Evtuguin2020).
Ellagic acid has been studied for its anti-parasitic effect in various studies. It showed significant therapeutic effects against malaria (Soh et al., Reference Soh, Witkowski, Olagnier, Nicolau, Garcia-Alvarez, Berry and Benoit-Vical2009), and leishmaniasis (Alves et al., Reference Alves, Arcanjo, Figueiredo, Oliveira, Viana, Coelho, Lopes, Gonçalves, Carvalho, Rizzo, Chaves, Mendonça and Carvalho2020). Accordingly, the current work was planned to assess the effectiveness of EA alone and combined with albendazole against Trichinella infection. To our knowledge, it is the first study that evaluates the effects of EA on trichinellosis.
Material and methods
Experimental animals and parasite
We used 60 Swiss albino male mice, 20–25 g in weight that were provided by Theodor Bilharz Research Institute, Egypt. T. spiralis larvae were taken from infected pig muscles in Cairo Slaughter House, Cairo, Egypt. The parasite was maintained at the Theodor Bilharz Research Institute. Larvae were recovered using acid-pepsin digestion method (Mayer-Scholl et al., Reference Mayer-Scholl, Pozio, Gayda, Thaben, Bahn and Nöckler2017). Mice were infected orally by 200–250 larvae per mouse in accordance with Dunn & Wright’s (Reference Dunn and Wright1985).
Ethical approval
Institutional Animal Care and Use Committee at Zagazig University approved the current study. The approval number is ZU-IACUC/3/F/30/2024.
Drugs
A commercial albendazole (Alzental) suspension (Eipico, Egypt) containing 20 mg/mL was utilised. Mice were given 50 mg/kg/d of albendazole orally for 3 days (Esmat et al., Reference Esmat, Abdel-Aal, Shalaby, Fahmy, Badawi, Elmallawany, Magdy, Afife and Shafi2021). Ellagic acid powder (Sigma-Aldrich, St Louis, MO, USA) was dissolved in saline and administered orally at 20 mg/kg/d for 7 days (Khan et al., Reference Khan, Khan, Azam, Allemailem, Alrumaihi, Almatroudi, Alhumaydhi, Azam, Khan, Zofair, Ahmad and Younus2021). Mice in combination group received the same doses.
Experimental design
There were two main groups of mice: G1 for the intestinal phase, which initiated treatment on the first day post infection (dpi) and sacrificed at eighth dpi; and G2 for the muscular phase, which started treatment on the 30th dpi and sacrificed at 42 dpi. Each group was divided into five subgroups (six mice each) as follows: (a) control non-infected; (b) control infected and non-treated; (c) infected and treated with EA; (d) infected and treated with albendazole; and (e) infected and received a combination of both.
Parasitological assessment
Adult worm isolation and counting
The small intestine was taken from mice in G1 and washed to remove intestinal contents. Then it was opened longitudinally, divided into small parts of 2 cm, and placed in normal saline at 37°C for 3 hours. Washing of intestinal parts was done three times. The collected fluids were then centrifuged at 1500 rpm for 5 minutes, after which the supernatant fluid was removed, and the sediment was examined to count adult worms using the dissecting microscope (Denham, Reference Denham1965; Issa et al., Reference Issa, El-Arousy and Abd EI-Aal1998).
Counting of muscle larvae
Muscle specimens were taken from each mouse of G2. The specimens were weighed and then digested by pepsin-HCl to make larvae out. The larval count was calculated per gram of muscle according to García et al. (2013).
For evaluating drug efficacy, the reduction percent (R%) was calculated: [(mean parasite count in controls - mean count in treated group)/ mean count in controls] ×100% (Saad et al., Reference Saad, Ashour, Abou Rayia and Bedeer2016).
Biochemical analysis
Serum biochemical analysis
Blood samples collection was done in Eppendorf tubes. After clotting, they were centrifuged at 1500 rpm for 15 min at 4°C to get the serum, which was stored at -20°C. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were estimated by a colorimetric method in serum by kits bought from Spinreact, Girona, Spain, according to the kit’s guidelines.
Collection and preparation of tissue samples
The intestinal and muscular samples were washed with ice-cold saline, divided into lesser parts, weighed, and then homogenised with a proper volume of 50 mM saline phosphate buffer, pH 7.4. The homogenate was centrifuged, and the supernatant was stored at -80°C until use for determination of nitric oxide (NO) level and catalase enzyme activity by a colorimetric method. Small tissue samples were immersed in RNA later and stored at -80°C for gene expression detection of interleukin-6 (IL-6) and interleukin-10 (IL-10) by using quantitative real-time polymerase chain reaction (qRT-PCR).
Assessment of oxidative stress markers in intestinal and muscle tissues
Nitric oxide level and catalase enzyme activity were measured using commercial kits from Biodiagnostic, Dokki, Giza, Egypt. Colorimetric test was performed as described by Montgomery and Dymock (Reference Montgomery and Dymock1961) and Aebi (Reference Aebi1984), respectively.
qRT-PCR for IL-6 and IL-10 gene expression in intestinal and muscle tissues (Schmittgen & Livak, Reference Schmittgen and Livak2008).
Total RNA was separated with TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RNA quality was tested by the A260/A280 ratio that was done by the NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies; Wilmington, Delaware, USA). The step of reverse transcription and complementary DNA (cDNA) formation was carried out by HiSenScriptTMRH [-] for the cDNA Synthesis Kit, INtRON Biotechnology, China. The gene expression analysis was performed by qRT-PCR using SYBR Green 2x Master Mix Green (QuantiTect SYBR Green PCR Kits, Qiagen). PCR was done using the Mx3005P RT-PCR System (Agilent Stratagene, USA) according to the manufacturer’s instructions to detect IL-6 and IL-10 relative expressions. The amplification procedure was as follows: 95°C for 5 min, followed by 35 cycles of 95°C for 30 s, 53°C for 30 s, and 72°C for 30 s, and a final incubation of 72°C for 5 min. GAPDH was used as a reference gene. Table 1 shows the primers used in our research.
Table 1. IL-6 and IL-10 primer sequences for qRT- PCR

Histopathological examination
Tissue specimens were taken from small intestine and muscles. They were fixed by immersion in 10% formalin, washed in water, then dehydrated in ascending grades of alcohols. The samples were then cleared in xylene and embedded in paraffin blocks. Microtome was used to make 5-μm thickness sections from these blocks. The hematoxylin and eosin method was used for staining (Banchroft et al., Reference Banchroft, Stevens and Turner1996).
Statistical analysis
SPSS version 25 (IBM, Armonk, NY, USA) was used for data analyses. Quantitative data were presented as mean ± SD. ANOVA (f) test was utilised for comparison between three or more groups having quantitative variables, comparison between groups was done using least significant difference method. A P value < 0.05 indicated statistical significance.
Results
Anti-parasitic effects
Treated groups G1c, G1d, and G1e revealed statistically significant reductions in adult counts in the small intestine compared to infected control G1b (P < 0.001). As well, there were significant differences between all groups concerning the mean adult counts except between G1d and G1e, as shown in Table 2.
Table 2. T. spiralis adult counts of different studied groups
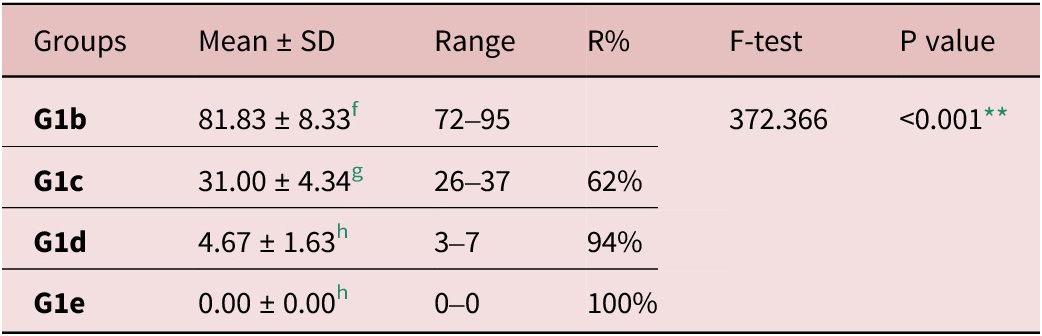
f, g and h Significant difference (P < 0.05) between any two groups within the same column have different superscript letter. No significant difference (P > 0.05) between groups with the same superscript.
R%: reduction percent;
** highly significant difference.
Regarding larval counts in muscular tissues, treatment of infected mice resulted in a significant decrease in mean numbers of encysted larvae, and the highest reduction was detected in G2e followed by G2d then by G2c. Additionally, there were significant statistical differences within the studied groups as seen in Table 3.
Table 3. Larval counts per gram muscle of different studied groups

f, g, h and i Significant difference (P < 0.05) between any two groups within the same column have different superscript letter. No significant difference (P > 0.05) between groups with the same superscript.
R%: reduction percent;
** highly significant difference.
Biochemical findings
Liver enzymes levels during the intestinal and muscular phases
A significant increase was determined in ALT and AST levels in infected control groups (G1b and G2b) when compared to corresponding non-infected control (G1a and G2a) (P < 0.001). Liver enzymes levels significantly decreased in EA treated groups (G1c and G2c) as well as combined treatment groups (G1e and G2e) in comparison with infected control. Their levels reached near normal in Ge groups (Table 4).
Table 4. Serum liver enzymes levels during intestinal and muscular phases of infection
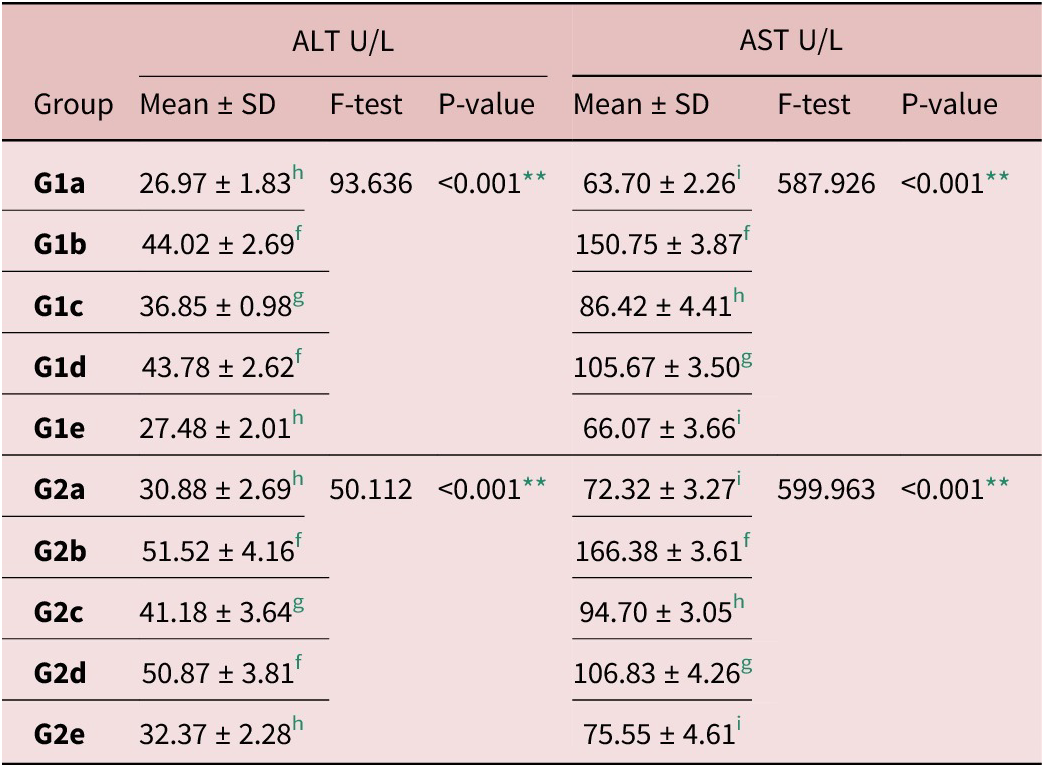
f, g, h and i Significant difference (P < 0.05) between any two groups within the same column have different superscript letter. No significant difference (P >0 .05) between groups with the same superscript.
** Highly significant difference.
Oxidative stress markers in intestinal and muscle tissues
T. spiralis infection (G1b and G2b) caused a statistically significant increase in the NO level in intestinal and muscular tissues in comparison to the normal control. All treated groups exhibited a significant decrease in NO levels in comparison with Gb groups. NO levels reached near normal in the combined treatment groups (G1e and G2e), as shown in Table 5.
Table 5. Oxidant/antioxidants in intestinal and muscle tissues homogenate
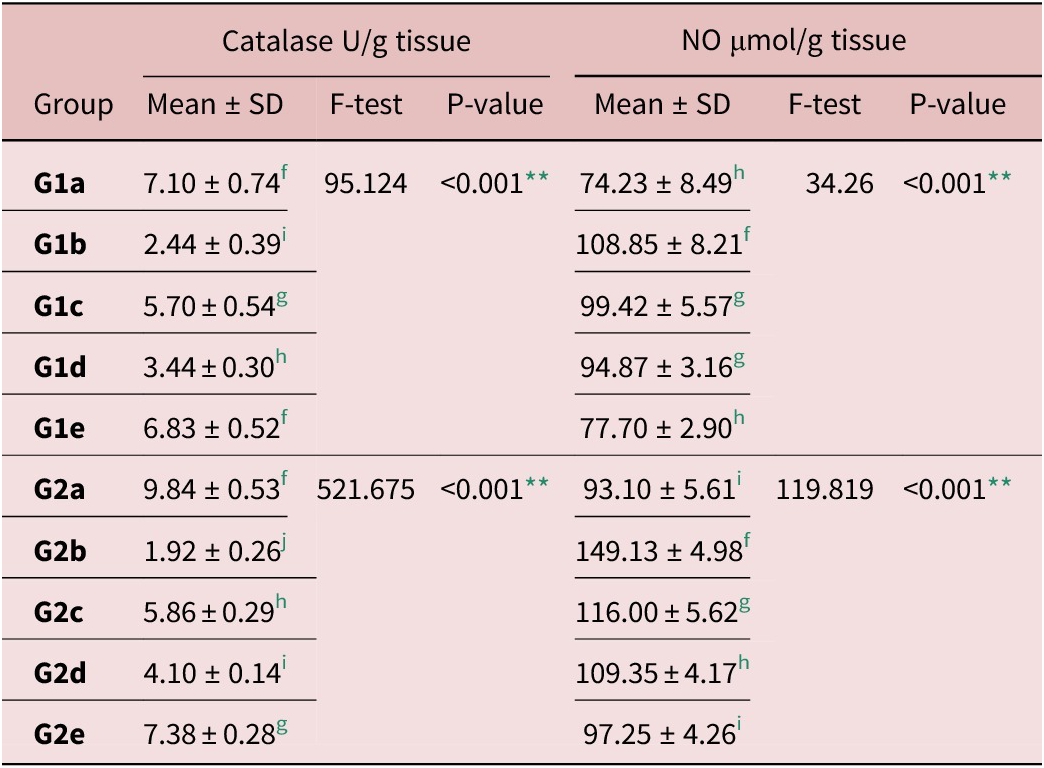
f, g, h, i and j Significant difference (P < 0.05) between any two groups within the same column have different superscript letter. No significant difference (P > 0.05) between groups with the same superscript.
** Highly significant difference.
On the other hand, catalase enzyme activity significantly decreased in infected control groups (G1b and G2b) when compared to normal controls (P < 0.001). Treatments induced an elevation of catalase enzyme activity, and the highest activity was induced by the combined treatment (Table 5).
IL-6 and IL-10 gene expression in intestinal and muscle tissues
IL-6 gene expression in infected control groups (G1b and G2b) significantly increased in relation to all other groups. There was a significant decrease in all treated groups compared to Gb groups. Combination therapy induced the maximum reduction of IL-6 gene expression in tissues (Table 6).
Table 6. Gene expression levels of IL-6 and IL-10 in intestinal and muscle tissues homogenate
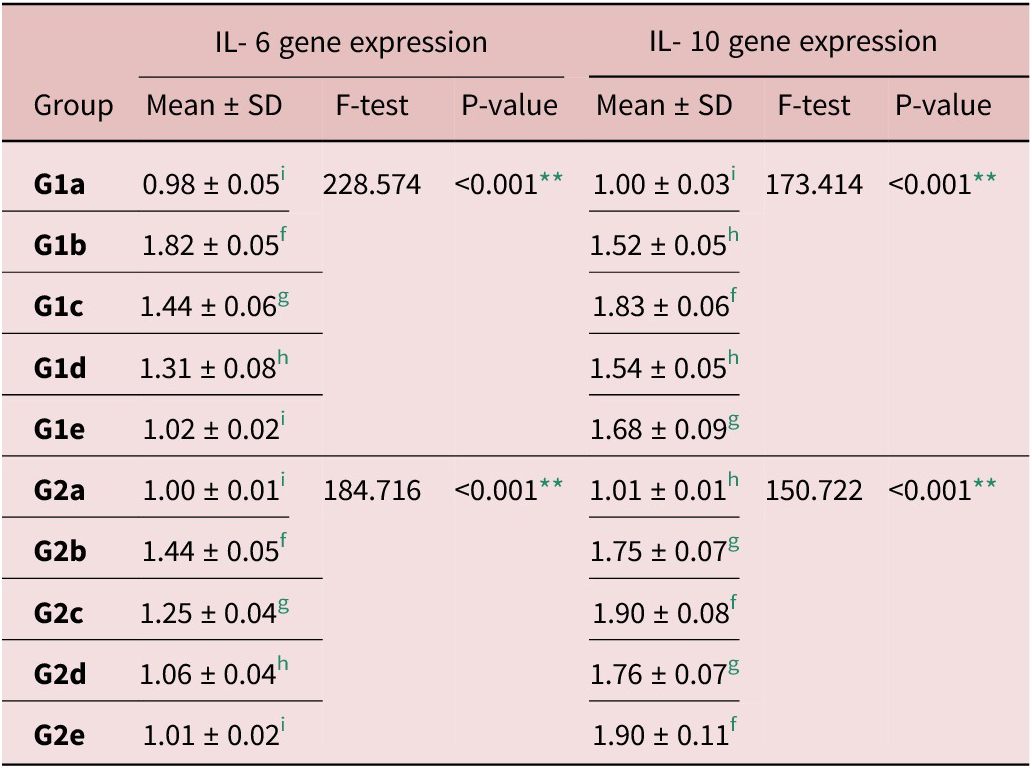
f, g, h and i Significant difference (P < 0.05) between any two groups within the same column have different superscript letter. No significant difference (P > 0.05) between groups with the same superscript.
** Highly significant difference.
Regarding IL-10 expression, there was a significant increase in its relative expression level in infected control (G1b and G2b) when compared to negative controls, with no significant difference with albendazole-treated groups (G1d and G2d). Additionally, there was a significant increase in IL-10 expression level in EA groups (G1c and G2c) as well as combined treatment groups (G1e and G2e) in comparison with other studied groups (Table 6).
Histopathological results
Intestinal sections from infected control G1b showed sections of T. spiralis adult worms, distorted villous pattern, and marked inflammatory infiltration of intestinal villi and crypts. G1c presented regenerating villi with moderate infiltration by mononuclear inflammatory cells. While G1d presented an improved intestinal architecture with normal crypts and mild villous inflammatory infiltrate. G1e showed the greatest improvement of intestinal tissues with nearly normal villous and crypt patterns with the absence of T. spiralis worms (Fig. 1).

Figure 1. Small intestinal sections of different groups (H&E ×200): (A) G1b showing T. spiralis adult worms (green arrow), distorted villous pattern (yellow arrows), and marked inflammatory infiltration (red arrow). (B) G1c showing regenerating villi (yellow arrow) with moderate mononuclear inflammatory infiltration (red arrows). (C) G1d showing villous regeneration (yellow arrows), normal crypts (green arrow) with mild villous inflammation (red arrow). (D) G1e showing nearly normal intestinal architecture with normal crypts (green arrow) and villi (yellow arrow).
Muscular pathological changes in infected control G2b showed many intact cysts and intense inflammation around encysted larvae with degenerative muscular changes. In comparison, G2c showed a reduction in the number of encysted larvae and inflammatory infiltration. G2d revealed degenerated cysts and fewer intact encysted larvae with moderate inflammatory cellular infiltration, whereas G2e exhibited mild inflammation and disintegrated larvae (Fig. 2).

Figure 2. Skeletal muscular sections of different groups (H&E ×200). (A) G2b showing disintegrated muscle tissue by many intact cysts (yellow arrows) and intense inflammation (red arrow). (B) G2c showed a reduction in the number of encysted larvae (yellow arrows) and mononuclear inflammatory infiltration (red arrow). (C) G2d showing degenerated cysts (black arrows) and fewer intact encysted larvae (yellow arrows) with inflammatory cellular infiltrate (red arrow). (D) G2e showing a disintegrated cyst (black arrow) with few inflammatory cells (red arrow).
Discussion
Several studies have demonstrated the significance of plant polyphenols as a class of antimicrobial compounds against bacteria, viruses and parasites. EA is a natural polyphenolic agent that has potent anti-inflammatory and antioxidant activities. It has free radical scavenging activities as it can transfer the phenolic H-atom to a free radical and is capable of accepting electrons from several substrates (Ceci et al., Reference Ceci, Lacal, Tentori, De Martino, Miano and Graziani2018; Ríos et al., Reference Ríos, Giner, Marín and Recio2018).
In the current study, EA and its combination with albendazole significantly reduced mean counts of T. spiralis intestinal adults and muscular larvae, with the highest percent reduction achieved by combination therapy. Additionally, EA was more effective in the intestinal phase than in the muscular phase. In accordance, Esmat et al. (Reference Esmat, Abdel-Aal, Shalaby, Fahmy, Badawi, Elmallawany, Magdy, Afife and Shafi2021) mentioned that Punica granatum peel extract, which is rich in antioxidants as EA, reduced T. spirals burden and inflammation and its anti-inflammatory effect was evident by the significant downregulation of CD4+ in tissues. This also was in agreement with Mohanty et al. (Reference Mohanty, Gupta, Maurya, Shanker, Pal and Bawankule2021), who found that oral administration of EA resulted in a significant suppression of malaria parasitemia with improved survival time. Soh et al. (Reference Soh, Witkowski, Olagnier, Nicolau, Garcia-Alvarez, Berry and Benoit-Vical2009) also reported that the combination of EA with current antimalarial medications presented a synergistic effect. Additionally, EA exhibited inhibitory effects on the growth and multiplication of various Babesia species in vitro and a reduction in B. microti parasitemia in vivo (Beshbishy et al., Reference Beshbishy, Batiha, Yokoyama and Igarashi2019). Moreover, EA was found to be effective against T. cruzi epimastigotes (Araújo et al., Reference Araújo, Lima, Rocha, Previtalli-Silva, Hardoim, Taniwaki, Calabrese, Almeida-Souza and Abreu-Silva2023).
In the present study, trichinellosis infected mice displayed a significant elevation in ALT and AST serum levels compared to non-infected ones. These results were consistent with the findings of Basyoni & Elsabah (Reference Basyoni and El-Sabaa2013) and Nasreldin et al. (Reference Nasreldin, Swilam, Abd-Elrahman and Abd El-ghaffar2022), who observed that liver damage caused by migrating larvae resulted in higher levels of liver enzymes in infected groups than normal ones. We found that albendazole induced a significant decrease in AST levels compared to the infected control, with no significant differences in ALT levels. Since AST is present in various tissues, including the liver, heart, and muscles (Center, Reference Center2007), albendazole’s reduction of the parasitic burden in these tissues likely resulted in less tissue damage, leading to a subsequent decrease in AST levels. Similar results were also obtained by Nasreldin et al. (Reference Nasreldin, Swilam, Abd-Elrahman and Abd El-ghaffar2022). This also aligned with Musa et al. (Reference Musa, Senocak, Borazan, Altas, Ozgonul, Sogut and Güldür2011), who observed a notable decrease in serum AST levels following albendazole treatment of Toxocara canis infection. EA as well as combination therapy enhanced liver functions by decreasing both ALT and AST levels. This decrease may be the result of the antioxidant properties of EA, which improve hepatic oxidative stress by raising antioxidant capacity. These findings concurred with those of Widyawati et al. (Reference Widyawati, Yuniarti and Lukiswanto2023), who reported that EA therapy improved hepatic functions.
The present study revealed that trichinellosis altered the oxidative status as the infected control groups showed higher NO levels and lower catalase enzyme activity in the intestinal and muscular tissues compared to the non-infected controls. Zhou et al. (Reference Zhou, Dong, Yang, He, Chen, Deng and Lan2015) found that the generation of NO during trichinellosis was a significant mediator of enteropathy because of the infection and may be a therapeutic target. We found that treatments resulted in a significant decline in NO levels and an elevation of catalase activity, and combination therapy induced the greatest improvement during both phases. Ateşşahín et al. (Reference Ateşşahín, Çeríbasi, Yuce, Bulmus and Çikim2006) reported that EA improved antioxidant activities as catalase and glutathione peroxidase enzymes. El-Shitany et al. (Reference El-Shitany, El-Bastawissy and El-desoky2014) also reported that EA induced protection against oxidative stress by decreasing NO, cyclooxygenase-2, and malondialdehyde expression and stimulating reduced glutathione production. Similarly, Mohanty et al. (Reference Mohanty, Gupta, Maurya, Shanker, Pal and Bawankule2021) found that EA therapy could protect from oxidative stress during Plasmodium infection. Albendazole was also found to improve the oxidative stress in T. spiralis-infected mice as it decreased inducible nitric oxide synthase and NO levels (Abdeltawab et al., Reference Abdeltawab, Abdel-Shafi, Aboulhoda, Wanas, Saad El-Din, Amer and Hamed2022), with better results when combined with antioxidants (Albogami, Reference Albogami2023).
In addition, we detected high expression levels of the pro-inflammatory cytokine IL-6 in intestinal and muscle tissue homogenates in infected controls, which was consistent with Munoz-Canoves et al. (Reference Munoz-Canoves, Scheele, Pedersen and Serrano2013), and Park et al. (Reference Park, Kang, Jo, Baek, Yu, Choi, Cha and Ock2018). Additionally, we detected a significant decrease in IL-6 in the EA and albendazole-treated groups compared to infected control, with levels returning to near normal by the combined therapy. These findings support the anti-inflammatory properties of EA. Umesalma and Sudhandiran (Reference Umesalma and Sudhandiran2010) detected negligible IL-6 expression in rats receiving EA, indicating that EA modulates inflammatory processes by inhibiting nuclear factor kappa B (NF-κB) activity and suppressing inflammatory cytokines. This also agreed with Mohanty et al. (Reference Mohanty, Gupta, Maurya, Shanker, Pal and Bawankule2021) who stated that EA induced a significant suppression of pro-inflammatory cytokines as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 in tissue homogenate of malaria-infected mice. Song et al. (Reference Song, Deng, Chen, Zhao, Li, Wu, Hou and Yi2024) also found that EA diminished IL-6 and TNF-α gene expression in the jejunum.
Our results showed a significant rise in IL-10 relative expression in the intestinal and muscular tissues of infected controls when compared to normal controls. Ding et al. (Reference Ding, Bai, Wang, Shi, Cai, Luo, Liu and Liu2017) detected that T helper 2 cytokines levels, including IL-4, IL-5, IL-10, and transforming growth factor-β, were increased in the small intestinal tissues of T. spiralis–infected animals. According to Park et al. (Reference Park, Kang, Jo, Baek, Yu, Choi, Cha and Ock2018), although IL-10 rose steadily and peaked at 42 days following infection, muscular inflammation started to decline at day 42. These results imply that IL-10 expression may affect the muscular anti-inflammation produced by trichinellosis. Additionally, we demonstrated that EA and combination therapy resulted in a significant elevation of IL-10 expression in comparison with other studied groups. Our results agreed with Favarin et al. (Reference Favarin, Teixeira, de Andrade, Alves, Chica, Sorgi, Faccioli and Rogerio2013), who found that EA treatment of acute lung injury increased IL-10 anti-inflammatory concentration in the bronchoalveolar lavage, indicating that EA can be used as an alternative therapy for inflammation control with less side effects than corticosteroids. Similarly, El-Shitany et al. (Reference El-Shitany, El-Bastawissy and El-desoky2014) stated that EA protects against inflammation by reducing NO, IL-1β, TNF-α, and NF-κB expression and enhancing IL-10 production.
Concerning histopathological examination of intestinal tissue sections, infected control revealed abnormal intestinal villi with marked inflammatory infiltration. This is mostly because of excretory-secretory proteins produced by adult worms that enable intestinal penetration (Khan, Reference Khan2008). These findings were in accordance with those of Elmehy et al. (Reference Elmehy, Saad, Gamal, El Maghraby, Arafa, Soliman, Elkaliny and Elgendy2021) and Salama et al. (Reference Salama, Mostafa, Abd El-Aal, Moawad, Hammad, Adel and Mostafa2022). Improvement in pathological alteration was noticed in all treated groups. EA therapy resulted in the regeneration of villi with fewer inflammatory infiltrations, and combination therapy induced the most pronounced improvement. EA was found to diminish the severity and extension of the intestinal damage and inflammation in animal models of colitis (Rosillo et al., Reference Rosillo, Sanchez-Hidalgo, C´ardeno and and Lastra2011; Marín et al., Reference Marín, Giner, Ríos and Recio2013). Khedr et al. (Reference Khedr, Gomaa, Mogahed, Gamea, Khodear, Sheta, Soliman, El Saadany and Salama2024) also informed that combining albendazole with curcumin displayed a significant potentiation against trichinellosis induced pathological changes better than albendazole alone. Concerning the muscular tissue examination of infected controls, there were many encysted larvae with intense inflammation and muscular degeneration. This was in agreement with Salama et al. (Reference Salama, Mostafa, Abd El-Aal, Moawad, Hammad, Adel and Mostafa2022) and Khedr et al. (Reference Khedr, Gomaa, Mogahed, Gamea, Khodear, Sheta, Soliman, El Saadany and Salama2024). EA displayed a considerable decrease in encysted larvae and a reduction in inflammatory infiltration, and combination therapy presented the best improvement. According to Labrecque et al. (Reference Labrecque, Lamy, Ame´lie Chapus, Samira Mihoubi, Durocher, Cass, Bojanowski, Denis Gingras and Be´liveau2005) and Ceci et al. (Reference Ceci, Lacal, Tentori, De Martino, Miano and Graziani2018), EA has significant antiangiogenic effects as it can inhibit VEGF receptors. That can interfere with larval nourishment, subsequently decreasing the larval count and controlling trichinellosis. Furthermore, its improving effects on the oxidative status and inflammatory response can help in enhancing the pathological alterations.
In conclusion, EA can ameliorate trichinellosis pathogenesis by suppressing proinflammatory cytokine production and oxidative stress and improving pathological alterations induced by T. spiralis infection. It also showed promising anti-trichinella therapeutic effects, which were greater in the intestinal phase, with synergistic activity when combined with albendazole. These results suggested the suitability of EA as a beneficial bioflavonoid for the treatment of trichinellosis.
Financial support
Nil.
Competing interest
The authors declare no conflict of interests










