INTRODUCTION
Cellulitis is a common inflammatory skin and soft tissue infection that is sometimes accompanied by purulent collections of pus (abscesses) [Reference Swartz1]. Skin and soft tissue infections are associated with 869 000 hospitalizations annually in the USA [Reference Edelsberg2]. Data from the National Ambulatory Medical Care Survey found that ambulatory care visits to emergency departments and physicians' offices for abscess/cellulitis infections had increased 88% from 8·6 million in 1997 to 14·2 million visits in 2005 [Reference Hersh3]. The true incidence and prevalence of cellulitis with and without abscess is difficult to estimate because the International Classification of Diseases, Clinical Modification, Ninth Revision (ICD-9-CM) does not distinguish between cellulitis and abscess.
Methicillin-resistant Staphylococcus aureus (MRSA) has become the predominant S. aureus strain causing community-associated skin infections in many places in the world, including the USA, with a prevalence exceeding that of methicillin-susceptible S. aureus (MSSA) [Reference Miller4, Reference Moran5]. MRSA skin infections are common, and in our medical centre [Reference Yang6] and region (Los Angeles County) [Reference Miller7, Reference Maree8] and in patients with suppurative infection, MRSA colonization is generally high (31–34%) [Reference Frazee9, Reference Baggett10] in contrast to much lower rates (1·2–3·0%) in the general population [Reference Chira and Miller11–Reference Bjornsdottir14]. Cellulitis is typically caused by S. aureus or group A streptococci [Reference Swartz1] but a recent systematic review of the literature on the aetiology of non-suppurative cellulitis demonstrated that S. aureus is the most commonly identified pathogen and almost twice as prevalent as group A streptococci [Reference Chira and Miller11]. While colonization with S. aureus is a recognized risk factor for S. aureus infection [Reference Safdar and Bradley12, Reference Hadley13], there are no data specifically on the relationship between non-suppurative cellulitis (for which skin cultures are rarely done) and S. aureus body colonization. Although case-control studies have found that obesity, previous history of infection, and homelessness are risk factors for non-suppurative cellulitis [Reference Bjornsdottir14–Reference Roujeau17], none have employed epidemiological surveys in their investigation or examined the role of S. aureus body colonization.
Given the frequency of S. aureus in non-suppurative cellulitis [Reference Chira and Miller11] and that MRSA colonization is common in patients with suppurative MRSA skin infection [Reference Yang6, Reference Frazee9, Reference Baggett10, Reference Safdar and Bradley12, Reference Lowy18], we hypothesized that patients hospitalized for non-suppurative cellulitis were more likely to be colonized with S. aureus and MRSA compared to matched controls. We examined this association and other hypothesized risk factors of patients hospitalized for non-suppurative cellulitis using a prospective case-control design.
METHODS
Study design
The investigation took place at Harbor–UCLA Medical Center, a 400-bed tertiary-care county hospital, from April 2008 to January 2009. Cases were identified via daily screening of the emergency department and urgent-care clinic by research coordinators. Enrolment was further enhanced with fliers asking emergency-department and urgent-care clinic personnel to contact the research coordinators if they saw a new patient with cellulitis. Cases were eligible for the study if they were being admitted to the medical centre. All diagnoses of non-suppurative cellulitis were confirmed by an attending dermatologist. Patients with skin infections that could be cultured (e.g. abscess, furuncle, carbuncle) or with documented or suspected deep or invasive skin infection (e.g. osteomyelitis, necrotizing fasciitis) were excluded. Controls were identified by screening in-patient wards for eligible patients. Two controls were matched to each case on age (±5 years), ethnicity, and gender. Eligible controls were excluded from participating if they had an active skin or S. aureus infection or had been hospitalized for >72 h. This investigation was approved by the Los Angeles Biomedical Research Institute at Harbor–UCLA Medical Center Institutional Review Board.
Data collection
A standardized instrument that used items from a previously published investigation of risk factors for skin infections was used to assess risk factors for cellulitis [Reference Miller4]. Based on data suggesting that nasal colonization alone is insensitive for detecting S. aureus body colonization [Reference Yang6], we collected nasal and inguinal culture swabs to test for the presence of S. aureus from cases and controls.
Microbiological tests and molecular analysis
Culture swabs were transported immediately to the microbiology laboratory and enriched in trypticase soy broth with 7% sodium chloride overnight at 35°C. The broth was subcultured to BBL CHROMagar S. aureus and MRSA plates (BD Diagnostics, USA) and incubated aerobically for 24 h at 35°C. Isolates were confirmed as S. aureus by the catalase test and StaphAureux test (Remel, USA). MRSA isolates, confirmed using CHROMagar MRSA plates, were subcultured twice for purity. DNA was extracted, digested with SmaI, and subjected to pulsed-field gel electrophoresis (PFGE) as previously described [Reference McDougal19]. DNA profiles were analysed using GelCompar software (Applied Maths, USA) and a reference database from the Centers for Disease Control and Prevention (CDC) containing the USA type strain patterns was used to assign the pulsed-field type (PFT).
Data analysis
Bivariate analysis was used to compare variables from the risk-factor questionnaire hypothesized to be associated with non-suppurative cellulitis in cases and controls. Bivariate analyses were assessed using odds ratios (OR) adjusted for 2:1 matching of controls to cases, 95% confidence intervals (CI), and the associated P values. All variables with a P value ⩽0·20 in the bivariate analysis were included in a multivariate logistic regression analysis predicting cellulitis using standard modelling procedures [Reference Kleinbaum, Klein and Pryor20]. Multicollinearity for the logistic regression model was assessed by condition indices and variance decomposition proportions using a macro developed for use with the SAS system. Backwards elimination was performed using the Likelihood Ratio test to find the best model. Models were examined for goodness of fit using the Hosmer–Lemeshow statistic. All variables were considered significant at the α=0·05 level. Data analyses were performed using SAS version 9.1.3 (SAS Institute, USA).
RESULTS
In total, 185 patients were enrolled (Fig. 1). Some enrolled patients were excluded from analysis for the following reasons: 29 cases were deemed as non-cellulitis skin conditions by the attending dermatologist; two cases had concomitant abscesses; one case was enrolled twice (the second instance was excluded from analysis); and three controls were incorrectly matched and not used in the analysis.
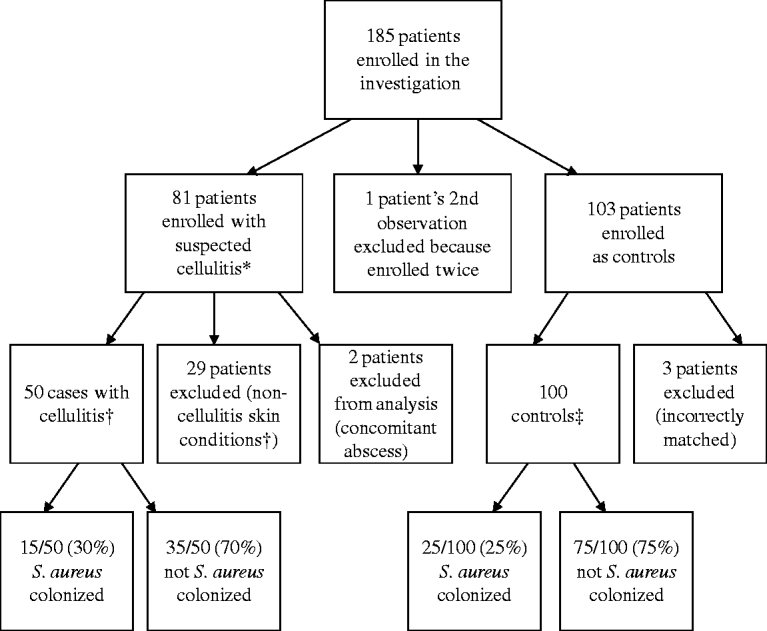
Fig. 1. Enrolment of subjects for prospective case-control study. * Diagnosed by the treating physician in the emergency department or urgent-care clinic. † Determined by the attending dermatologist. ‡ Matched by age, race/ethnicity, and gender (see text for details).
The data for 50 non-suppurative cellulitis cases along with 100 matched controls were analysed. The mean age of cases was 40 years (median 42 years, range 2–83 years), 58% were male, and 56% were Hispanic, 16% of cases were African-American, 16% were Caucasian, and 12% were of other race/ethnicity. A representative case is shown in Figure 2.
Fig. 2. Representative patient with non-suppurative cellulitis (of the foot).
The results for the bivariate analysis are summarized in Table 1. Thirty percent (15/50) of cases and 25% (25/100) of controls were colonized with S. aureus (P=0·51). MRSA colonization was 6% (3/50) in cases and 3% (3/100, OR 2·0, 95% CI 0·41–10·0, P=0·39) in controls. In the bivariate analysis, diabetes (OR 3·3, 95% CI 1·4–7·8), use of antibiotics in the previous 12 months (OR 4·0, 95% CI 1·003–16·0), and homelessness in the previous 12 months (OR 6·3, 95% CI 2·1–19·3) were all found to be associated with cellulitis (P⩽0·05). Previous cellulitis was more common in cases (11/50, 22%) compared to controls (1/100, 1%) (we were unable to calculate ORs due to the sparse data in the individual strata). Using an unmatched analysis, previous cellulitis was a predictor of acute non-suppurative cellulitis (OR 28·7, 95% CI 3·6–229·6).
Table 1. Bivariate analysis of non-suppurative cellulitis cases compared to controls
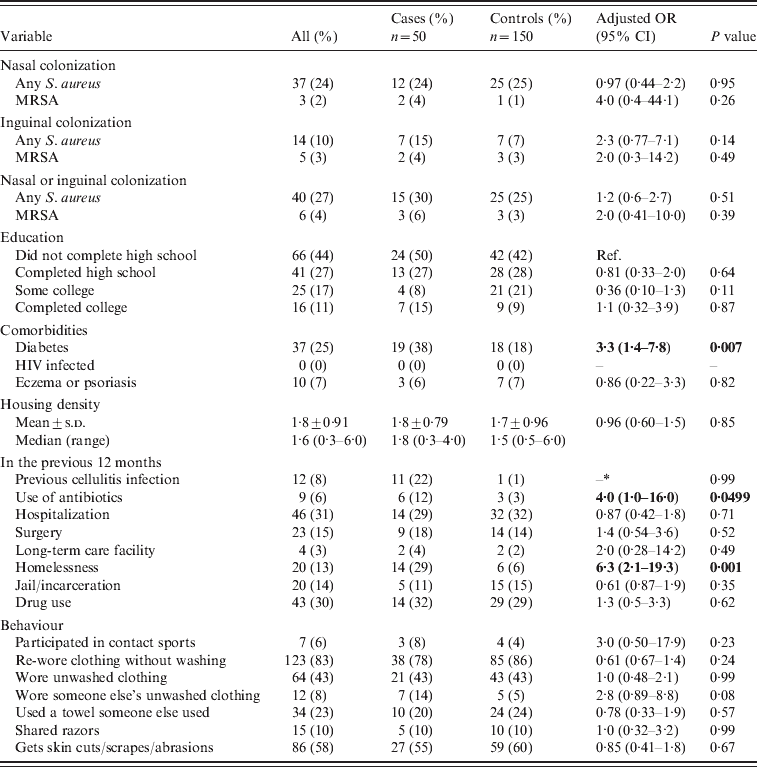
OR, Odds ratio; CI, confidence interval; Ref., reference group; –, cannot be calculated.
Bold values indicate statistically significant variables.
Not all subjects answered all questions.
* Data too sparse to be calculated.
When examining predictors of S. aureus colonization, we found no significant interaction between S. aureus colonization and diabetes, antibiotic use, or homelessness. In the multivariate logistic regression model of cellulitis, diabetes (OR 3·5, 95% CI 1·4–8·9, P=0·01) and homelessness (OR 6·4, 95% CI 1·9–20·9, P=0·002) were found to be independently associated with non-suppurative cellulitis.
We recovered eight MRSA isolates for PFGE typing [from three cases (n=4) and from three controls (n=4), Table 1] and all isolates were strain type USA300 (Fig. 3). The two patients with MRSA cultured from their nasal and inguinal areas yielded indistinguishable strains.
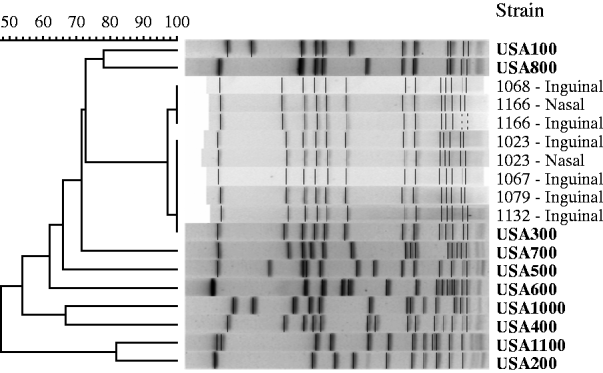
Fig. 3. Pulsed-field gel electrophoresis of MRSA isolates from cases of non-suppurative cellulitis and uninfected controls, and profiles of reference MRSA strains (in bold).
DISCUSSION
Cellulitis is a common clinical problem resulting in substantial clinical morbidity, hospitalization, and cost. In our prospective matched case-control study, we found that S. aureus colonization is present in 30% of patients with non-suppurative cellulitis. This percentage is similar to that of the general population [Reference Gorwitz21] and was not significantly different from our control population. No significant association was found between cellulitis and either S. aureus or MRSA colonization of the nares or inguinal region. Diabetes and homelessness were significant risk factors for non-suppurative cellulitis.
Our study was performed in an area of MRSA endemicity where most suppurative skin infections are caused by MRSA [Reference Miller4–Reference Yang6]. The lack of association between non-suppurative cellulitis and either S. aureus or MRSA colonization was not consistent with our a priori hypothesis. Our findings are potentially inconsistent with current thought regarding the pathogenesis of soft tissue infections, i.e. that soft tissue infections are frequently preceded by S. aureus colonization [Reference Safdar and Bradley12, Reference Ellis22, Reference Shet23]. Colonization findings in our patients with non-suppurative cellulitis may be distinct from that of suppurative skin infections for several reasons. The most common aetiology of non-suppurative cellulitis remains unknown, as investigations using needle aspirations and punch biopsies of cellulitis usually find no culturable pathogens [Reference Chira and Miller11]. However, in suppurative skin infections, a swab culture will usually (>75%) reveal a bacterial infection and most commonly, S. aureus [Reference Moran5]. It is possible that S. aureus colonization may not precede non-suppurative skin infections such as cellulitis. Alternatively, the aetiology of culturable, suppurative cellulitis is different from non-culturable, non-suppurative cellulitis and the latter is infrequently caused by S. aureus, despite this being the most commonly culturable pathogen [Reference Chira and Miller11] (i.e. non-S. aureus pathogens are much more difficult to isolate).
Although not sampled in this study, S. aureus pharyngeal colonization is more common than previously recognized [Reference Mertz24–Reference Widmer, Mertz and Frei26] and may be important in the pathogenesis of S. aureus infections. Of interest, a recent matched case-control study of non-suppurative cellulitis in Finland found that <10% of cellulitis cases were pharyngeally colonized with S. aureus. The investigators also found a significant difference between case (7%) and control (0%) pharyngeal colonization with group G Streptococcus (P⩽0·04) [Reference Siljander27]. However, there was no examination of risk factors or nasal or inguinal colonization in that investigation.
In our study, non-suppurative cellulitis was independently associated with diabetes and homelessness. However, three other prospective case-control investigations, conducted in Europe and focusing only on lower-limb non-suppurative cellulitis, did not find an association between cellulitis and diabetes [Reference Bjornsdottir14, Reference Dupuy15, Reference Roujeau17]. A retrospective case-control study from the USA, which also focused on lower-limb cellulitis, found a significant association between non-suppurative cellulitis and homelessness [Reference Lewis16]. Our investigation appears to be the first study to find an association between diabetes and non-suppurative cellulitis, although other studies have found associations between diabetes and suppurative soft tissue infections [Reference Miller28, 29].
There are limitations to this study. First, the patients were enrolled from a single centre and findings may not be generalizable to other populations. However, the patient population at our institution is similar to many community hospitals and is ethnically diverse. Second, we also relied on patients to self-report risk factors. Patients may be reticent to acknowledge less socially acceptable risk factors such as incarceration, substance use or high-risk sexual behaviour. Nevertheless, in a previous investigation at this institution using this instrument, risk factors that may be considered socially undesirable were significantly associated with MRSA risk [Reference Miller4], suggesting that the survey has validity and limited bias. Third, we did not examine pharyngeal colonization in our study. However, the increased yield from determining pharyngeal S. aureus colonization in addition to nasal and inguinal colonization is not well understood. Fourth, our controls were chosen from hospitalized patients, which may suggest that controls were not selected independently of exposure to S. aureus. Nevertheless, this concern is mitigated because patients with active skin or S. aureus infection or who had been in the medical centre for >72 h were ineligible to be controls. Additionally, there were no significant differences between cases and controls in hospitalization in the previous 12 months (29% vs. 32%, P=0·71).
There are many strengths to our investigation. First, while almost all other studies on S. aureus colonization and skin infections group together non-suppurative cellulitis with abscesses or other suppurative processes, we focused on investigating the association between colonization and non-suppurative (bland) cellulitis. If the pathogenesis of non-suppurative cellulitis is different from suppurative skin infections as suggested by other studies [Reference Spellberg30], then distinguishing risk factors for non-suppurative cellulitis will be important in understanding the distinct pathogenesis of this infection. Second, while many other studies focus solely on colonization of the anterior nares, we screened for colonization at the anterior nares and the inguinal region, increasing the sensitivity of S. aureus detection [Reference Yang6]. A third strength of our investigation is that controls were matched at a 2:1 frequency on age, gender, and ethnicity. This increases the statistical efficiency of the investigation compared to other case-control studies [Reference Rothman and Greenland31]. Fourth, a dermatologist confirmed all cases of cellulitis for this investigation. Between 20% and 35% of patients admitted to the hospital with cellulitis are misdiagnosed by treating physicians and actually have non-infectious processes (such as stasis dermatitis, contact dermatitis, or non-specific dermatitis) [Reference David32]. In our study, 29/81 patients admitted for cellulitis were excluded due to misclassification of diagnosis (i.e. they were given an incorrect diagnosis of cellulitis). Given the limitations of accurately diagnosing cellulitis, our method probably significantly minimized case misclassification.
In conclusion, we found that despite MRSA being the predominant cause of suppurative skin infections at our centre [Reference Miller4] and MRSA colonization being common (37%) in hospitalized patients with suppurative MRSA skin infections [Reference Yang6], MRSA colonization is uncommon in patients with non-suppurative cellulitis (6% in the current study). Further, the low level of MRSA colonization in cases of non-suppurative cellulitis was similar to that of controls and the general population [Reference Gorwitz21, Reference Kenner33, Reference Malik34]. This suggests that MRSA may not be a common cause of non-suppurative cellulitis. If this hypothesis is proven, empirical therapy of non-suppurative skin infections may not require anti-MRSA therapy. Other studies of cellulitis aetiology in areas of MRSA endemicity as well as investigations of S. aureus colonization and risk factors in patients with non-suppurative cellulitis would help further to clarify the pathogenesis of non-suppurative cellulitis. Hopefully such findings will lead improved interventions to prevent and treat this common skin infection.
ACKNOWLEDGEMENTS
Financial support was provided in part by an unrestricted grant from Fallon Medica Inc. and in part by General Clinical Research Center (GCRC) Grant M01-RR00425 National Center for Research Resources. We thank Mellie Badar and the Harbor–UCLA Medical Center for their assistance with this investigation. We also thank the patients and their families for participating in this investigation.
DECLARATION OF INTEREST
None.






