INTRODUCTION
Hepatitis B virus (HBV) infection is a worldwide health problem. About one billion people in the world have been infected by HBV and 200 million of them have become chronic hepatitis B surface antigen (HBsAg) carriers. The world is conceptually divided into regions of high, intermediate and low endemicity. In the intermediate endemic regions (including Turkey), in which the overall prevalence of infection, as judged by serological markers of HBV, ranges from 20% to 60%, and carriers of HBsAg from 2% to 10% of the general population [Reference Hsu1, Reference Greate and Giusti2].
HBV transmission from HBsAg carrier mothers and fathers to their children is a very important route of infection. Apart from parenteral and vertical transmission, HBV may be transmitted through sexual, child-to-child or household personal contact [Reference Kim and Ahn3]. Around 90% of the infants of HBsAg-positive carrier mothers become HBsAg carriers [Reference Stevens4]. In this context, it is not surprising that several members of the same household may have evidence of HBV infection. In such cases, transmission of the virus is thought to have occurred by intra-familial contact [Reference Erol5–Reference Zervou11]. Recently, by sequencing the viral genome, it has been shown that the HBV genome of index patients bears 100% sequence homology with their infected family members [Reference Lobato10].
HBV transmission through familial contact may differ in the prevalences due to cultural and religious aspects. This has been the first large-scale study ever conducted in eastern Turkey that was designed to investigate the rate of the intra-familial spread of HBV. We conducted a 4-year prospective study in Sivas, Turkey. The aim of the present study was not only to determine the prevalence of HBsAg in children of HBV-infected parents but also to identify all HBSAg-positive family members to protect as many children, in the present and future, as possible.
MATERIAL AND METHODS
The present study was carried out with the participation of 2113 family members (1205 children, 453 mothers, and 455 fathers) at Sivas SSK Hospital. They were screened for HBV markers between September 2001 and March 2005. The first member (mother or father who stayed with the children in the same house) presenting to the hospital as a HBsAg carrier was defined as the index case. The index cases were found to be positive for HBsAg during the last 6-month period. A detailed clinical examination was performed for each index case to assess the clinical status. A total of 2224 family members agreed to participate in the study, constituting 95% of the surviving family members of the 454 index cases. The remaining 5% were not available for testing because they were either living away or refused to donate blood. Families who had no child were not included in our study. A total of 154 (31·9%) of the index cases were asymptomatic carriers who were detected accidentally, and the remainder presented with chronic hepatitis.
Presence of any of the markers of infection, i.e. HBsAg/anti-HBs/anti-HBc, indicated exposure to HBV infection. The presence of anti-HBs alone is a marker of vaccination and anti-HBc total marker positivity is indicative of past exposure to HBV.
The Ethics Committee of KSU Medical Faculty approved the study protocol, and informed consent was obtained from all the participating families. All subjects who consented to participate in the study were asked to complete a questionnaire on general data, risk factors for HBV infection and kinship to the index case. Therefore, the results presented here are not based on samples obtained from a population-based study, but based on biased clinical samples.
Venous blood samples and markers detection
The blood samples were collected, stored in the laboratory and frozen at −20°C after aliquoting their sera until they were required for study. HBsAg, hepatitis B surface antibody (anti-HBs) and hepatitis B core antibody (anti-HBc) were tested by commercially available enzyme-linked immunosorbent assay (ELISA) kits (Organon Teknika, Boxtel, The Netherlands) according to the manufacturer's instructions. Samples positive for HBsAg were tested for HBeAg and antibody to HBeAg (anti-HBe) using an Organon Teknika kit. The sera of all index cases, and their family members were tested for HBsAg, anti-HBs, anti-HBc, HBeAg and anti-HBe.
Statistical analysis
Data were expressed as mean values±s.d., median and range or as number of subjects and percentages. χ2 test was used for comparison of numerical data. P values <0·05 were considered statistically significant. Analyses were performed by using SPSS software, version 9.05 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
Four hundred and fifty-four index cases, 259 male and 195 female subjects (age range 18–76 years, mean 39±11) were assessed in the study. In 10 (2·2%) of the index cases, the onset of infection and duration of carrier state were noted whereas, in the remainder (97·8%) the exact onset of infection could not be determined.
Twenty-two subjects (4·8%) were HBeAg positive, 417 subjects (91·9%) were anti-HBe positive and the remaining 15 subjects of the index cases (3·3%) were HBeAg and anti-HBe negative.
The prevalence of all HBV markers and HBsAg among family members of index cases was 50·5% and 30·5% respectively. HBsAg carrier rate was higher among fathers (61%) than mothers (47%) (P<0·05).
Serological markers of HBV infection in the family members are shown in Figure 1.

Fig. 1. Serological markers of hepatitis B virus infection in the family members. ■, HBsAg(+);![]() , anti-HBc total(+);
, anti-HBc total(+);![]() , anti-HBs(+); □, any HBV marker (+).
, anti-HBs(+); □, any HBV marker (+).
Hepatitis B serology of different age groups is given in Figure 2. The prevalence of HBsAg was different in various age groups (P<0·01), and reached the highest rate in the 41–50 years age group (P<0·05).

Fig. 2. Hepatitis B serology of different age groups. ■, HBsAg(+);![]() , anti-HBc total(+);
, anti-HBc total(+);![]() , anti-HBs(+); □, any HBV marker (+).
, anti-HBs(+); □, any HBV marker (+).
Of the 491 children of 177 families with an HBsAg-positive mother, 97 (19·7%) were HBsAg positive, and of the 624 children of 241 families with an HBsAg-positive father, 34 (5·4%) were HBsAg positive. However, of the 90 children with both parents HBsAg positive, 24 (26·6%) were HBsAg positive. The children of mother index cases had higher rates of HBsAg compared with the children of father index cases (P<0·01) (Fig. 3).

Fig. 3. HBV infection in the family and children according to HBsAg status of the parents (M, mother; F, father). ■, HBsAg(+) children (n=number of children).
The rate of HBsAg positivity in families with a single child was 4·8%, but in families with 2–5 children, it was 12·2%. Furthermore, it was found to be 26% in families with >5 children (P<0·001) (Table 1).
Table 1. Rate of carriage in children based on the number of children in the family
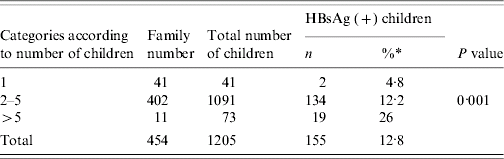
* The percentages were calculated according to row.
DISCUSSION
In Turkey, the reported prevalence of HBsAg carriage varies from 4% to 9% in the general population. The overall prevalence of HBV infection as judged by serological markers, such as HBsAg, anti-HBs and anti-HBc, ranges from 20% to 60% [Reference Mıstık, Balık, Kılıçturgay and Badur12]. According to the prevalence of infection, Turkey may be included in the intermediate endemic regions. It is difficult to state clearly what the main transmission routes of HBV infection in Turkey are [Reference Taşyaran, Kılıçturgay and Badur13]. Although the time sequence of transmission among family members was unclear, either parent of a family who was determined as a HBsAg carrier was defined as an index case in this study. Therefore, the definition of an index case is arbitrary, and may be controversial. It is, however, difficult to confirm the sequence of events.
In our study, the prevalence of HBsAg was different among age groups, but high carriage (∼50%) HBV infection was most common in those aged ⩾31 years and reached the highest rate in the 41–50 years age group. Moreover, a high carriage rate in the 40–50 years age group has been reported in previous studies from Turkey [Reference Kaygusuz14, Reference Kilic15]. Similar studies showed that the prevalence of HBV markers increased progressively with age [Reference Kim and Ahn3, Reference Toukan6]. Therefore, this finding points to an early exposure during life in the Anatolian region. Anti-HBs was the highest in the 0–10 years age group in our study. This can be explained by the universal vaccination campaign against HBV. In this study, the rate of HBsAg positivity was 30·5% among overall cases. Moreover, the rate of any HBV positivity was 50·5%. The rate of HBsAg positivity was found to be similar but the rate of any HBV positivity was higher than in a previous study conducted by Erol et al. in the same region of Turkey [Reference Erol5]. The prevalence of HBsAg and any HBV marker from different countries varies [Israel (16·6% and 67·8%), India (19·4% and 57·1%), Brazil (24·3% and 70·5%), Greece (15·8% and 42·1%) and Iran (11·9% and 60·5%), respectively [Reference Bisharat8–Reference Zervou11, Reference Alizadeh16]) (Table 2). The present study showed evidence of HBsAg positivity in around 30% of the family contacts and exposure to HBV (any HBV marker) in more than half of the family contacts of HBV-infected index patients. Thus our data highlight intra-family clustering as an important mode of HBV transmission in Turkey. Multiple family members in small dwellings facilitate close and intimate interpersonal physical contacts. Presence of HBV DNA has been documented in saliva, sweat and urine of HBV-infected persons, making it highly probable that the family may provide an excellent setting for virus transmission through any of these vehicles, either by non-percutaneous or by overt percutaneous means [Reference Kim and Ahn3, Reference Lobato10, Reference Martinson, Wiegle and Royce17–Reference Zhevachevsky19]. This pattern of familial aggregation of HBV infection has been noted elsewhere in the world [Reference Kim and Ahn3, Reference Toukan6, Reference Abdool7, Reference Lok20, Reference Tan21].
Table 2. According to various literatures, rates of HBsAg and Any HBV markers in the family members of index cases who were HBsAg carriers
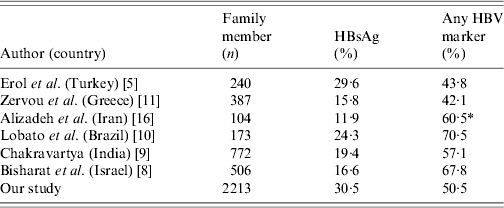
* HBe/Anti-HBe tests were not performed.
Of the children of families with an HBsAg-positive mother 19·7% were HBsAg, and of the families with both parents HBsAg positive 26·6% were HBsAg positive. However, of families with an HBsAg-positive father 5·4% were HBsAg positive. The frequency of HBsAg in children with HBsAg-positive mothers was 10·4% in the Brazilian Amazon region [Reference Lobato10]. Aribas et al. showed that the children of mother index cases had higher rates of HBsAg than those of father index cases [Reference Aribas22]. It was also reported in the study that rate of transmission from the mother index case to the children was 40·6%, and that of transmission from both HBsAg-positive parents to the children was found to reach up to 57·1%. A study from Erzurum, Eastern Turkey has also found similar results on the importance of the mother in HBV transmission [Reference Erol5]. The father-to-child HBV transmission rate was given as 9·4% by Franks et al. [Reference Franks23] and 13% (50% of which was confirmed by sequence analyses) by Takegoshi et al. [Reference Takegoshi and Zhang24]. Our result in the present study was 5·4% and underestimated both studies. This situation may be explained with the fact that in Turkish families, fathers mostly work outdoors, while mothers spend most of their time in childrearing and household work. This cultural and customary backdrop means that intimate contact occurs between the mother and child as well as among the siblings for prolonged periods of time and facilitates horizontal transmission of HBV in these settings [Reference Chakravartya9–Reference Zervou11, Reference Dumpis25]. HBV transmission within families is greater, if they have an HBsAg-positive mother rather than an HBsAg-positive father [Reference Porres26]. Thus, the mother is an important figure of virus contamination in intra-familial transmission in Turkish families.
In our study, the rate of HBsAg positivity in the household increases proportionately with the number of children in the family. Karagoz et al., in the east of Turkey, reported that in families of five or more individuals, there had been no statistical difference in positivity [Reference Karagoz and Akbulut27]. However, as is also shown in our study, Aribas et al., found a significant relationship between family size and HBs positivity in central Turkey [Reference Aribas22]. Szmuness et al. [Reference Szmuness28], Toukan et al. [Reference Toukan6] and Ramia et al. [Reference Ramia29] showed that family size affected HBV transmission in the household and increased proportionately with the number of children in the family. But there are some controversial reports concerning the role of family size in the transmission of HBV infection [Reference Milas30, Reference Williams31].
Our results suggest that intra-familial childhood horizontal transmission (especially mother-to-child) is important for HBV transmission in the community, and highlights the need for screening of adult siblings and mothers of adult HBsAg carriers in addition to their spouses and children. These findings strongly emphasize that an HBV vaccination schedule, along with the investigation of all members of the family for the presence of HBV markers, should be offered immediately and early in life to all family members of chronic carriers. Health education regarding modification of such factors might constitute a very cost-effective ancillary approach to prevention of hepatitis B in Turkey in coordination with the regulations of WHO. Further studies are necessary to establish exactly the route of dissemination of HBV in Turkey.
DECLARATION OF INTEREST
None.







