Incidence and epidemiology of pneumococcal meningitis (PM) have markedly changed worldwide with the use of pneumococcal conjugate vaccines (PCV) in children aged <2 years [Reference Martin1–Reference Bogaert, De Groot and Hermans4]. After introduction of PCV7, a massive decrease of incidence in PM was first observed [Reference Martin1, Reference Tin Tin Htar3, Reference Dubos5]. A few years later, a slower decrease was observed and even a small increase in a few centres [Reference Alexandre6], due to serotype replacement with the emergence of non-vaccine serotypes [Reference Weinberger, Malley and Lipsitch7]. After switching from PCV7 to PCV13 in late 2010 in France, PM prevalence decreased again in the whole population [Reference Levy8]. But serotype replacement was still observed with the emergence of new serotypes in nasopharyngeal carriage [Reference Yildirim9], and in PM cases. The real impact of PCV13, based on exhaustive data, is not currently available.
The aim of this study was to determine the impact of PCV13 on the incidence of PM in northern France. Secondary objectives were to compare serotype characteristics and PCV status of those PM cases.
This new descriptive, retrospective, multicentre cohort study included all cases of PM in patients aged <18 years admitted in one of the 18 paediatric centres in northern France (Nord-Pas-de-Calais region comprising almost 1 million children) between 1 January 2008 and 31 December 2013. The methodology was similar to two previous studies [Reference Dubos5, Reference Alexandre6]. PM was defined by cerebrospinal fluid (CSF) pleocytosis (⩾7 cells⁄mm3) and a pneumococcal infection in either CSF or blood, documented by culture, polymerase chain reaction (PCR) or antigen detection. Patients with haemorrhagic CSF (blood cell count >10 000/mm3) were excluded since distinguishing between PM or pneumococcal sepsis alone was impossible. Two periods were defined: a pre-PCV13 period between 2008 and 2010 when children were vaccinated with PCV7 and a post-PCV13 period during which PCV13 was exclusively used. In the national vaccination schedule PCV was recommended at 2, 3, and 4 months and, since 2009, at 2, 4 months as primary vaccination, with a booster dose at age 12 months.
In each centre, a local coordinating investigator, belonging to our hospital network, informed the study investigators of cases with a standardized questionnaire. Demographics (age, sex, vaccination status, medical history, risk factors for invasive pneumococcal disease), clinical data [use of antibiotics before PM, coma, seizure, other neurological features, haemodynamic failure, therapeutics used (i.e. antibiotics, corticosteroids), outcome], laboratory data (white blood cell and neutrophil counts in CSF) and microbiological findings (CSF Gram staining, CSF and blood cultures with serotypes, antigens and/or PCR in blood and/or CSF) were recorded for each patient. Vaccination status was obtained from the health book of each child and was defined as (1) complete, (2) incomplete vaccination status or (3) no PCV indication at the time of PM diagnosis, according to national guidelines. All these data were extracted from medical files in each paediatric unit and from the bacterial meningitis registry of each hospital's microbiology laboratory. In each local hospital, this database was cross-checked with suitable discharge codes as primary or associated diagnosis, according to the 10th International Classification of Diseases, to enable exhaustivity. Demographic estimates were collected from the Regional Observatory for Health. The National Reference Centre for Streptococcus pneumoniae provided the serotypes responsible for PM. For this kind of retrospective observational study, patients/parents are systematically informed that personal data can be used for statistical analyses (and can refuse this), but neither approval from an ethics committee nor patients'/parents' consent is required in France. However a CNIL (National Commission for informatics and liberties) authorization was obtained for the use of individual data.
Epi Info 6·04 software (Centres for Disease Control and Prevention, Atlanta, USA) was used for analyses. The total number of PM cases was determined by cross-checking databases. Patients' characteristics were described. The annual distribution of cases and the corrected incidence of PM were assessed by capture–recapture analysis, globally and according to age groups, with their 95% confidence intervals (CI). The incidence was corrected by an exhaustive estimation of cases through this multiple database analysis. Incidences were compared between 2008 and 2013 and between pre- (2008–2010) and post- (2011–2013) PCV13 periods by χ 2 tests. The distribution of annual pneumococcal serotypes was classified as either vaccine or non-vaccine serotypes and compared with the vaccination status.
After cross-checking the two databases, 62 cases of PM were identified over the study period [median age 57·4 (±63·9) months, male:female ratio 2·2], with 53% of children aged <2 years. Clinical characteristics at admission were high-grade fever (100%), coma (42%), seizure (21%) and other neurological features (motor disability or nerve palsy) (40%). Acute otitis media was associated with meningitis in 18% of cases, acute sinusitis in 6·4% and acute pneumonia in 3%. Antibiotic treatment within 48 h before admission was prescribed in 34% of cases. Fifty-five percent were admitted to the intensive care unit because of haemodynamic failure (29%) and⁄or coma (42%). Neurological sequelae were found in 48% of cases. The mortality rate at 30 days was 9·7% (95% CI 2·3–17·0).
Fifty-six patients were common to the two databases, four identified by the hospital database only and two by the discharge code database only. After capture–recapture analysis, the corrected number of PM cases was of 62·33. A decrease of the PM corrected incidence was observed in the population of children aged <18 years from 1·8/100 000 in 2008 to 0·8/100 000 in 2013, which was not significant (2·1 fold-decrease, 95% CI 0·9–5·4, P = 0·07). This decrease was significant for children aged <2 years, from 11·9/100 000 in 2008 to 1·9/100 000 in 2013 (6·4 fold-decrease, 95% CI 1·4–41, P = 0·01) (Table 1). Similar results were obtained when comparing PM incidences between pre- and post-PCV13 periods with a statistically significant decrease for children aged <2 years [7·32/100 000 (95% CI 4·39–10·25) to 2·78/100 000 (95% CI 0·96–4·60), P = 0·01) (see Supplementary material).
Table 1. Pneumococcal meningitis (PM) episodes reported in each database and corrected incidence of PM in children aged <18 years yearly and between the pre- and post-PCV13 periods in northern France (by capture–recapture method over two databases for each age group)
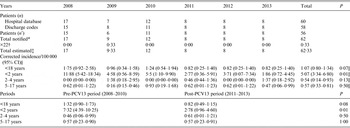
CI, Confidence interval; n’, patients identified in both databases.
* Total number of cases notified in almost one of the two databases.
† ×22 = estimation of patients missed = (number of cases only identified by hospital database × number of cases only identified by discharge codes)/number of cases identified in both databases.
‡ Number of cases only identified by hospital database + number of cases only identified by discharge codes + number of cases notified in both databases (n’) + ×22;
§ Total estimated × 100 000/total population in the considered ages.
|| Comparison between the years 2008 and 2013.
Of the 50 serotypes available (12 missing; seven in pre- and five in post-PCV13 period), a 2·1-fold decrease of PCV13 serotypes was observed between the two periods, which was not significant (P = 0·34) (Supplementary material). Two vaccinated patients had three PM episodes each during the study period, with non-vaccine serotypes: a 5-year-old child with Goldenhar disease with three PM episodes all in 2013 secondary to a post-surgical osteomeningeal fistula being finally identified. Another child born in 1998 was diagnosed with three PM episodes, in 2005, 2008 and fatal episode in 2010 without identification of osteomeningeal fistula or immunodeficiency. Besides these two patients, three others had an osteomeningeal fistula (post-surgical, n = 1, post-head trauma, n = 2) and three had miscellaneous underlying diseases (congenital heart disease, n = 2, premature baby, n = 1).
The vaccination status was unknown for four children. For the others, 21 (37%) had no PCV indication because they were born before 2002 (n = 16) or aged <2 months (n = 5). Of the 37 children with a PCV recommendation, 68% were up-to-date for PCV and 84% had at least one dose of PCV.
When considering the vaccination status and serotypes, three children without medical history that did not receive any PCV dose (9%) could have been avoided. A 9-month-old child had a 9 V-PM in 2008 and died; a 5-month-old child had a 19A-PM in 2011, with severe neurological sequelae (tetra-pyramidal syndrome and epilepsy); a 3-month-old child had a 7 F-PM in 2012 without sequelae. A 7-year-old child with cardiac failure had a PCV7-related PM episode in the post-PCV13 period; he was vaccinated with two PCV7 injections and one Pneumo23 injection 3 years before 7 F-PM was diagnosed (serotype included in Pneumo23).
This retrospective study identified 62 cases of PM between 2008 and 2013 in children aged <18 years in the study area. After capture–recapture analysis from two sources, a decrease was observed in the global incidence between the pre- and post-PCV13 periods, statistically significant for children aged <2 years (P = 0·01). No PCV failure was observed.
A trend in decrease of PM cases after the introduction of PCV13 has been seen elsewhere. In France between 2001 and 2012 a decrease in the prevalence of PM was observed in children aged <2 years (28% decrease between 2009 and 2012) [Reference Levy8]. A study over 50 years (1963–2011) in England has shown a decrease in incidence of admission rate for PM in children aged <15 years (from 4·45 to 2·03/100 000) after the introduction of PCV13 [Reference Martin1]. To date, our study is the first to provide a corrected incidence of PM cases in children in the PCV13 era.
There are some limitations to our study. First is its retrospective design; however, exhaustive case ascertainment was reached by use of two different data sources, which were completely independent. Although the region has a population of about 1 million children aged <18 years, the number of cases in this study is limited (n = 62), which can explain the absence of variation in PM incidence in the other age groups and the non-significant reduction of serotypes 7 F and 19A in the post-PCV13 period. Although each S. pneumoniae responsible for invasive disease should be centralized, 20% of serotypes were missing because the strains were not sent to the national reference centre. This can also explain the absence of significant difference in PCV13 serotypes between the two periods. Only confirmed cases of PM were considered. The global PM incidence may therefore be partially underestimated by not considering haemorrhagic CSF (n = 1) and pre-treated undocumented meningitis. However, neither diagnosis methods nor relevant medical practices changed over the study period. These cannot explain our results either. Finally, the PCV coverage was high and stable (>90%) during the study period [Reference Martinot10], as confirmed by the number of PCV sold each year (Supplementary material).
No PCV13 failure was observed in this study. In the post-PCV13 period, three patients could have been protected if vaccinated. All the others had either only one dose of the vaccine, which was not enough to give protection, or were too young (<2 months) or too old at the time of PCV recommendation to be vaccinated with PCV13. In conclusion, after the introduction of PCV13, a decrease in the incidence of PM cases in children aged <2 years in northern France was observed. Nevertheless, careful monitoring of PM incidence must be continued in order to detect the emergence of new serotypes and adapt the PCV strategy in the future.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S095026881500179X.
ACKNOWLEDGEMENTS
We are grateful to the microbiologists in the hospitals of northern France and paediatricians involved in the Hospital Network for Evaluating the Management of Common Childhood Diseases: Drs Akitani (Seclin), Audry-Degardin (Valenciennes), Besset (GHIC Lille), Chrigua (St Omer), Cixous (Roubaix), Delepoulle (Dunkerque), Devouge (Arras), Dhaoui (Cambrai), Grailles (Tourcoing), Glowacki (Armentières), Louf (Montreuil/Mer), Martinet (Béthune), Mikem and Najafzadeh (Maubeuge), Neut (Boulogne), Penel (Lens), Racoussot (Douai), Renault (Calais); and staff from the Departments of Medical Information of each centre, particularly: Drs Adam, Boulanger, Bricoteau, Coevet, Dufossez, Houyengah, Kyndt, Lafitte, Nuttens, Paradis, Paty, Perard, Quentin, Schaffar, Taleb, Tilloy, Vandenbussche, Vasseur, Verin, Wemeau, Youssef.
DECLARATION OF INTEREST
None.




