Introduction
Schizophrenia is a chronic, severe, and often disabling mental illness characterized by deficits in thought processes, perceptions, and emotional responsiveness. 1 , Reference van Os and Kapur 2 The treatment of schizophrenia typically requires a multidisciplinary approach that involves both psychosocial and psychopharmacological interventions.Reference van Os and Kapur 2 Over the past decade, advances and innovations in antipsychotic medications have enabled physicians to tailor treatment regimens according to patients’ individual needs. For example, patients who have difficulty adhering to daily oral medication may benefit from using long-acting injectable (LAI) antipsychotic therapies, which provide therapeutic plasma concentrations over several weeks and ensure adherence.Reference Pandina, Lindenmayer and Lull 3 – Reference Nasrallah 5 Although LAIs have been available for several years, clinical trials comparing the efficacy and safety of LAIs and oral antipsychotics have produced inconsistent resultsReference Chue, Eerdekens and Augustyns 6 – Reference Kishimoto, Robenzadeh and Leucht 9 and therefore warrant further study.
The atypical once-monthly LAI antipsychotic paliperidone palmitate (PP) is a nanocrystal formulation of paliperidone that dissolves slowly after intramuscular injection. 10 The efficacy, safety, and tolerability of PP have been evaluated extensively in patients with schizophrenia in several short-term (ie, ≤6 month) explanatory trials.Reference Pandina, Lindenmayer and Lull 3 , Reference Pandina, Lane and Gopal 4 , Reference Hargarter, Cherubin and Bergmans 11 – Reference Alphs, Bossie, Sliwa, Fu, Ma and Hulihan 22 Fewer studies have examined the longer-term (ie, >6 month) efficacy and safety of PP,Reference González-Rodríguez and Catalán R, Penadés 18 , Reference Hough, Lindenmayer and Gopal 23 – Reference Zhang, Si and Chiou 28 and because these trials were primarily explanatory in design, their results do not necessarily reflect real-world situations.Reference Alphs, Mao, Rodriguez, Hulihan and Starr 27 Additional long-term studies that evaluate real-world outcomes are needed to better understand both the efficacy and effectiveness of PP in patients with schizophrenia.
The Paliperidone Palmitate Research in Demonstrating Effectiveness (PRIDE) studyReference Alphs, Mao, Rodriguez, Hulihan and Starr 27 , Reference Alphs, Benson, Cheshire-Kenny, Lindenmayer, Mao and Rodriguez 29 is a 15-month, prospective, randomized study comparing the effects of PP with those of daily oral antipsychotics (ie, haloperidol, perphenazine, olanzapine, aripiprazole, quetiapine, risperidone, and paliperidone) on treatment failure in a trial designed to reflect real-world schizophrenia patients, treatments, and outcomes. PRIDE is unique in that it incorporates both explanatory (efficacy) and pragmatic (effectiveness) design elements. PRIDE included patients traditionally excluded from randomized trials, such as those with a history of incarceration and comorbid substance abuse; allowed flexibility in treatment and management decisions; and included a range of real-world consequences as endpoints (ie, arrest/incarceration, psychiatric hospitalization, suicide, discontinuation due to inadequate efficacy or intolerability, treatment supplementation due to inadequate efficacy, or increased psychiatric services to prevent hospitalization). The primary results of PRIDE showed that treatment with once-monthly PP significantly delayed treatment failure compared with daily oral antipsychotics in the overall study population (hazard ratio [HR]: 1.43; 95% confidence interval [CI]: 1.09–1.88; log-rank p=0.011); the median event time was 190 days longer in the PP group, and there was a 43% reduction in risk of treatment failure relative to oral antipsychotics.Reference Alphs, Benson, Cheshire-Kenny, Lindenmayer, Mao and Rodriguez 29
The PRIDE study’s randomization scheme provides a sampling basis for exploratory comparisons of once-monthly PP to classes of oral antipsychotic medications. We hypothesized that the significant delay in treatment failure observed with once-monthly PP would be observed when compared with subgroups of conventional oral antipsychotics (ie, haloperidol and perphenazine) and atypical oral antipsychotics (ie, aripiprazole, olanzapine, quetiapine, paliperidone, and risperidone). Additionally, we sought to compare the efficacy and safety of once-monthly PP with oral paliperidone and oral risperidone to examine outcomes following treatment with different delivery methods of the same molecule platform (ie, LAI versus oral delivery of risperidone and its active metabolite, paliperidone).
Methods
Study design
The detailed methodology has been published elsewhere.Reference Alphs, Mao, Rodriguez, Hulihan and Starr 27 , Reference Alphs, Benson, Cheshire-Kenny, Lindenmayer, Mao and Rodriguez 29 In brief, PRIDE (NCT01157351) was a randomized, prospective, open-label, event-monitoring board–blinded, parallel-group study that compared once-monthly PP and oral antipsychotics on treatment failure in subjects with schizophrenia and a history of incarceration. The study included a screening phase of up to 2 weeks, followed by a 15-month randomized, open-label treatment phase.Reference Alphs, Mao, Rodriguez, Hulihan and Starr 27 The study was conducted between May 5, 2010, and December 9, 2013, at 50 sites across 25 U.S. states and Puerto Rico.Reference Alphs, Benson, Cheshire-Kenny, Lindenmayer, Mao and Rodriguez 29 The study protocol was approved by each site’s institutional review board and was conducted in accordance with the ethical principles established by the Declaration of Helsinki.
Participants
Adults aged 18 to 65 years with a current diagnosis of schizophrenia (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria 30 that was confirmed by the M.I.N.I. International Neuropsychiatric Interview, version 6.0Reference Sheehan, Lecrubier and Harnett Sheehan 31 ) were eligible for study participation. Subjects must have been taken into custody by the criminal justice system ≥2 times in the previous 2 years, with ≥1 of these events leading to incarceration; have been released from most recent custody within 90 days of the screening visit; and be willing to use a once-monthly LAI antipsychotic.Reference Alphs, Benson, Cheshire-Kenny, Lindenmayer, Mao and Rodriguez 29 All subjects provided written informed consent.
Interventions
Study interventions included paliperidone palmitate and oral antipsychotic medications.Reference Alphs, Benson, Cheshire-Kenny, Lindenmayer, Mao and Rodriguez 29 Seven oral antipsychotic medications were available to participants: haloperidol, perphenazine, olanzapine, aripiprazole, quetiapine, risperidone, and paliperidone. Before random treatment assignment, subjects reviewed these oral medication options with their physicians to determine acceptability based on prior experience. Up to 6 oral antipsychotic medications could be deselected by the subject or the physician, and reasons for deselection were documented.
Randomization
An equipoise stratified randomization schemeReference Lavori, Rush and Wisniewski 32 was used for treatment assignment.Reference Alphs, Benson, Cheshire-Kenny, Lindenmayer, Mao and Rodriguez 29 The equipoise strata were defined by the sets of acceptable oral antipsychotic medications selected by subjects and their physicians before randomization. Subjects were randomized within their equipoise stratum in a 1:1 ratio to treatment with flexibly dosed once-monthly PP (78–234 mg) or a flexibly dosed oral antipsychotic that was randomly selected from the oral medications in the equipoise stratum. For example, subjects who selected all 5 atypical antipsychotics as acceptable treatment options were randomized according to a randomization schedule allocated for the atypical antipsychotic stratum, subjects who selected the 2 conventional antipsychotics were randomized according to a randomization schedule allocated for the conventional antipsychotic stratum, and so on. The randomization scheme provided a sampling basis for comparing multiple treatment options. For example, to compare PP versus conventional antipsychotics, subjects who were randomized would be pooled from the strata that included 1 or more conventional antipsychotics.
Study medications
Flexible monthly maintenance doses of once-monthly PP were given according to the product label in a dose range of 78–234 mg (50–150 mg equivalents); the recommended target maintenance dose was 156 mg.Reference Alphs, Benson, Cheshire-Kenny, Lindenmayer, Mao and Rodriguez 29 Oral antipsychotic doses were selected and adjusted within the dose range of the package insert; occasional dosing outside the range specified in the package insert was allowed. The use of nonantipsychotic psychotropic medications (ie, mood stabilizers, antidepressants, anxiolytics, or hypnotics) was permitted if clinically indicated as concomitant therapy; however, monotherapy with the assigned study drug was encouraged. Subjects who discontinued study treatment or experienced treatment failure and did not withdraw consent could continue to be followed through month 15 of the treatment phase.
Study end points
For this analysis we used the primary study endpoint, time to treatment failure, which was defined as any of the following events: arrest or incarceration, psychiatric hospitalization, suicide, discontinuation of treatment due to inadequate efficacy (in the investigator’s opinion), treatment supplementation with another antipsychotic due to inadequate efficacy, discontinuation of treatment due to safety or tolerability, or increase in psychiatric services to prevent imminent psychiatric hospitalization.Reference Alphs, Benson, Cheshire-Kenny, Lindenmayer, Mao and Rodriguez 29 First treatment failure events were initially assessed by site investigators, and each event was subsequently reviewed and adjudicated as determined by an independent event-monitoring board that was blinded to individual subject treatment assignment. Exploratory analyses of time to treatment failure compared the following: (1) once-monthly PP versus daily conventional oral antipsychotics (haloperidol or perphenazine), (2) once-monthly PP versus daily atypical oral antipsychotics (olanzapine or aripiprazole or quetiapine or paliperidone or risperidone), and (3) once-monthly PP versus daily oral paliperidone plus risperidone.
Statistical analyses
Statistical analyses were the same as those used for the primary analysis.Reference Alphs, Benson, Cheshire-Kenny, Lindenmayer, Mao and Rodriguez 29 The intent-to-treat (ITT) population, defined as all randomly assigned subjects who received ≥1 dose of their study treatment, was used for efficacy and safety analyses. Event-free probabilities were estimated using the Kaplan–Meier method; treatment differences were assessed using a log-rank test based on ITT analysis set; HRs and 95% CIs were estimated using Cox proportional hazards regression models, with randomly assigned treatment as a fixed factor. The number needed to treat (NNT) was calculated as the inverse of absolute risk reduction, defined as the difference in estimated event rates at month 15 between treatment groups. The differences across subgroups regarding adverse event (AE) outcomes were reported descriptively. The differences in demographics and baseline variables between treatment subgroups were assessed using the Wilcoxon rank-sum test for continuous variables or Fisher’s exact test for categorical variables. No adjustment was made for multiplicity.
Results
Reasons for deselection of oral antipsychotic medications
Reasons for the deselection of oral antipsychotic medications prior to the start of the study are shown in Table 1. Haloperidol (59.9%) and perphenazine (39.4%) were the most commonly deselected oral antipsychotics prior to randomization, primarily because of extrapyramidal symptom (EPS)–related AEs. Paliperidone was deselected less than any other oral antipsychotic (7.7%). For atypical oral antipsychotics, the most common deselection reasons were weight gain and other.
Table 1 Reasons for deselection of oral antipsychotic medications at study start in total study population (n=444) (intent-to-treat population)
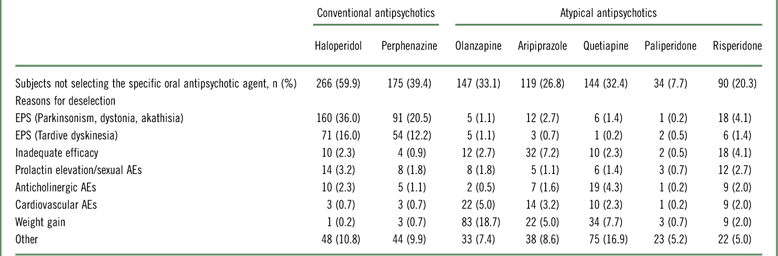
Abbreviations: AEs, adverse events; EPS, extrapyramidal symptoms.
Patient disposition and baseline characteristics
In the primary analysis, 450 subjects were randomly assigned to PP (n=230, 51.1%) or oral antipsychotics (n=220, 48.9%) and 444 subjects were included in the ITT population.Reference Alphs, Benson, Cheshire-Kenny, Lindenmayer, Mao and Rodriguez 29 Of these, 226 (50.9%) received PP and 218 (49.1%) received oral antipsychotics (183 [83.9%], atypical oral antipsychotics; 35 [16.1%], conventional oral antipsychotics) (Figure 1). Sample sizes for the PP versus conventional oral antipsychotic comparison, the PP versus atypical oral antipsychotic comparison, and the PP vs paliperidone plus risperidone comparison are also shown in Figure 1. Baseline demographics and clinical characteristics were generally similar (ITT population) (Table 2). The mean (SD) daily doses (mg) of prescribed oral antipsychotics were 8.2 (5.33), 16.5 (8.81), 13.3 (6.44), 15.3 (5.89), 339.9 (180.35), 3.6 (1.61), and 6.6 (2.44) for haloperidol, perphenazine, olanzapine, aripiprazole, quetiapine, risperidone, and paliperidone, respectively. The mean (SD) monthly PP dose was 181.3 (34.19) mg.
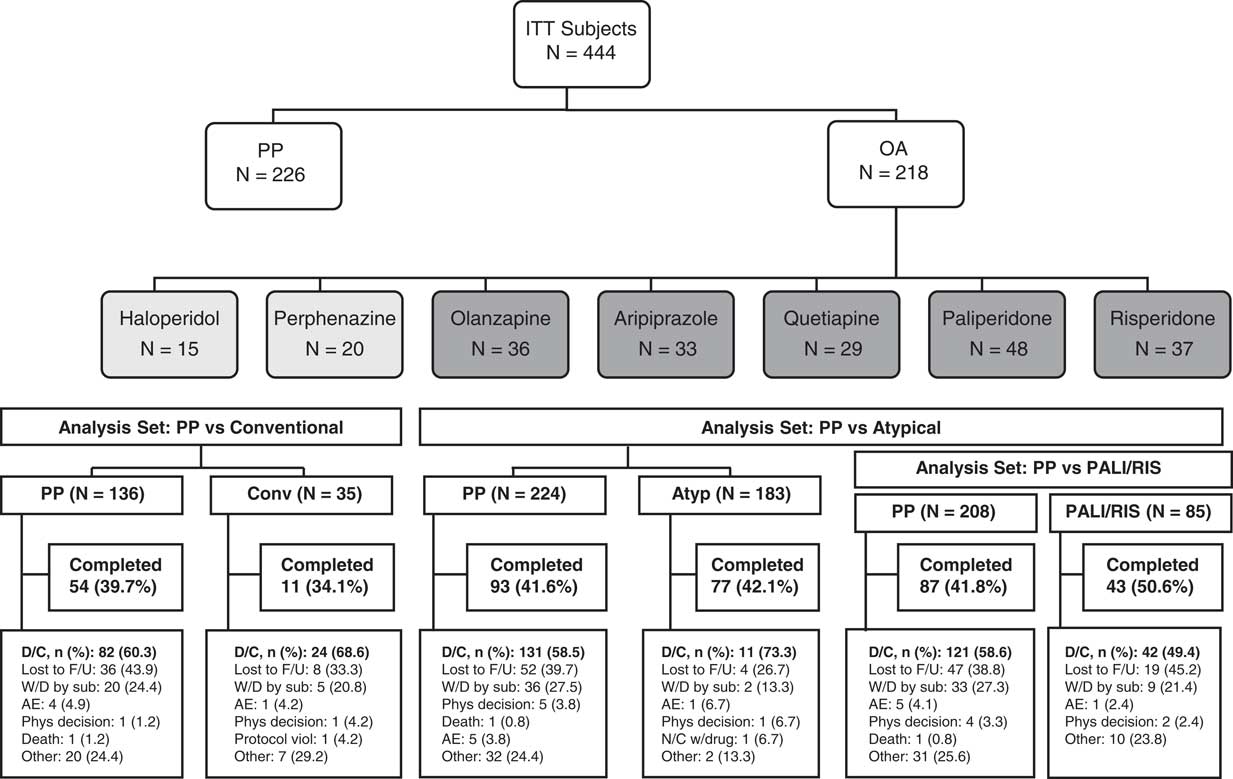
Figure 1 Study flow diagram and analysis sets. Abbreviations: AE, adverse event; AP, antipsychotic; Atyp, atypical antipsychotic; Conventional/Conv, conventional antipsychotic; D/C, discontinued; F/U, follow-up; N/C, noncompliance; OA, oral antipsychotic; PALI/RIS, paliperidone/risperidone; Phys, physician; PP, paliperidone palmitate; sub, subject; viol, violation; W/D, withdrawal.
Table 2 Demographic and baseline characteristics (intent-to-treat population)
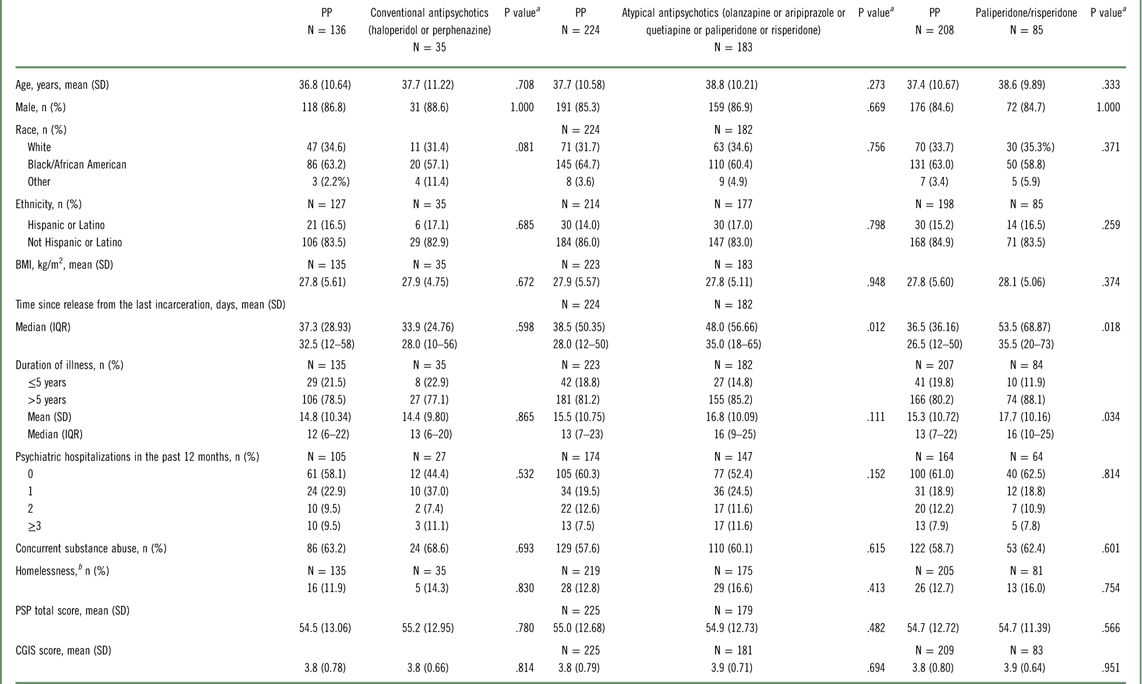
Abbreviations: BMI, body mass index; CGIS, Clinical Global Impressions–Severity; IRQ, interquartile range; PP, paliperidone palmitate; PSP, Personal and Social Performance scale; SD, standard deviation.
a P values are from Wilcoxon rank-sum tests for continuous variables and from chi-square tests for categorical variables.
b Homelessness is defined as living on the streets or in an emergency shelter for the homeless since the time of release from jail.
First treatment failure
Once-monthly PP versus conventional oral antipsychotics
In total, 136 subjects receiving once-monthly PP and 35 subjects receiving conventional oral antipsychotics were included in this analysis. Treatment failures occurred in 62 (45.6%) subjects in the PP group and in 19 (54.3%) subjects in the conventional oral antipsychotic group. The estimated event rate at month 15 was 57.0% in the PP group and 66.0% in the conventional oral group (NNT=11). The risk for treatment failure was 34% higher with conventional oral antipsychotics (HR: 1.34; 95% CI: 0.80–2.25; p=0.262) than with once-monthly PP (Figure 2A). The median number of days to treatment failure was 302 days for PP and 142 days for conventional oral antipsychotics.
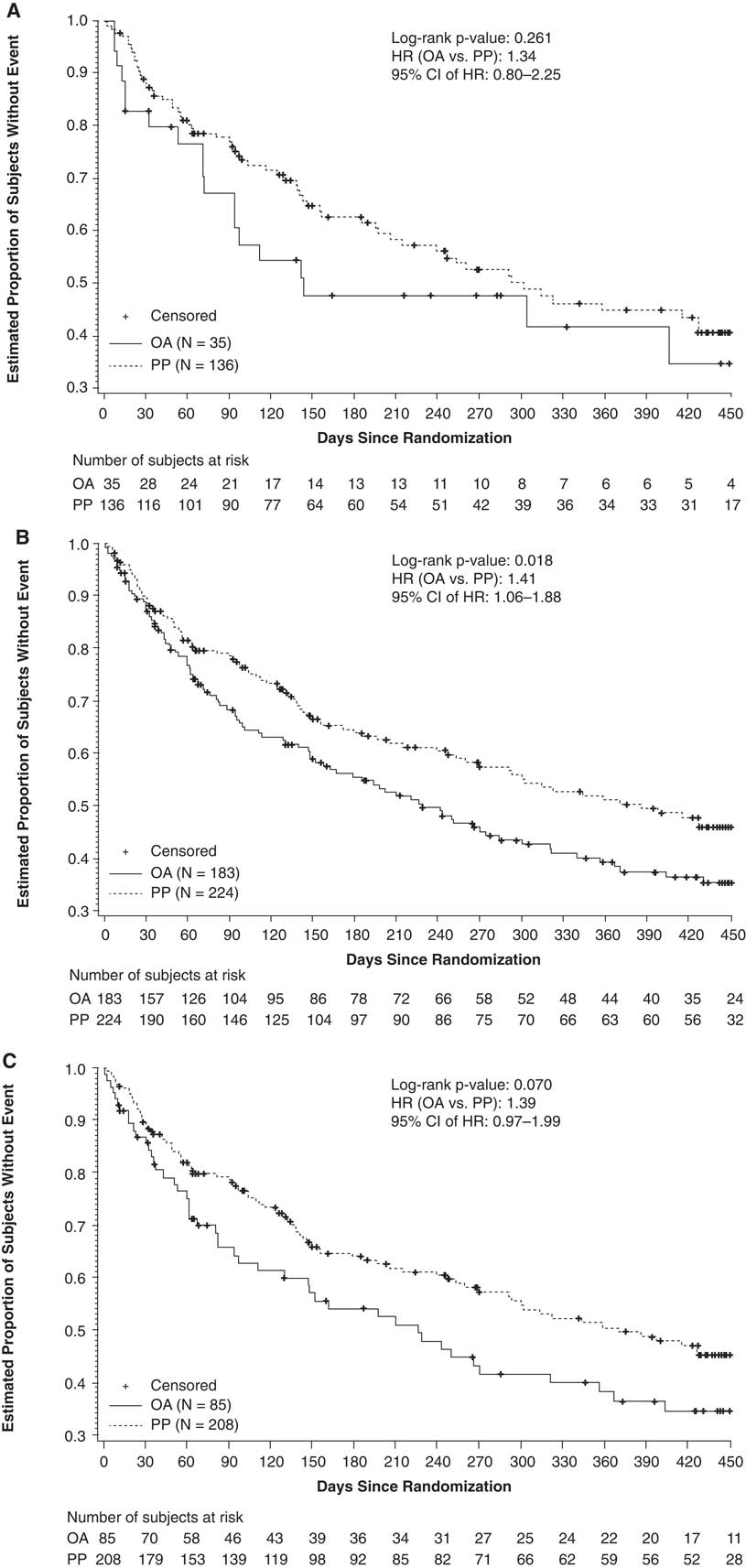
Figure 2 Kaplan–Meier estimates of time to first treatment failure. (A) PP vs conventional antipsychotics (haloperidol or perphenazine). (B) PP vs atypical OAs (paliperidone, risperidone, olanzapine, aripiprazole, or quetiapine). (C) PP vs paliperidone/risperidone. Abbreviations: CI, confidence interval; HR, hazard ratio; OA, oral antipsychotic; PP, paliperidone palmitate.
Once-monthly PP versus atypical oral antipsychotics
In total, 224 subjects receiving once-monthly PP and 183 subjects receiving atypical oral antipsychotics were included in this analysis. Eighty-nine subjects (39.7%) in the PP group and 98 (53.6%) subjects in the atypical oral antipsychotic group had a treatment failure event. The estimated event rate at month 15 was 52% in the PP group and 63% in the atypical oral antipsychotic group (NNT=9). The risk for treatment failure was 41% higher with atypical oral antipsychotics (HR: 1.41; 95% CI: 1.06–1.88; p=0.019) than with PP (Figure 2B). The median number of days to treatment failure was 428 days for PP and 229 days for atypical oral antipsychotics.
Once-monthly PP versus paliperidone/risperidone
A total of 208 subjects receiving once-monthly PP and 85 subjects receiving oral paliperidone/risperidone were included in this analysis. Treatment failures occurred in 85 (40.9%) subjects in the PP group and in 46 (54.1%) subjects in the paliperidone/risperidone group. The estimated event rate at month 15 was 53% in the PP group and 64% in the oral paliperidone/risperidone group (NNT=9). The risk for treatment failure was 39% higher in the paliperidone/risperidone group (HR: 1.39; 95% CI: 0.97–1.99; p=0.071) than with PP (Figure 2C). The median number of days to treatment failure was 416 days for PP and 229 days for paliperidone/risperidone.
Once-monthly PP versus individual oral antipsychotics
Comparisons of once-monthly PP to individual oral antipsychotics lacked the power to detect statistically significant differences. However, there was a trend toward better efficacy with once-monthly PP, including versus oral delivery of paliperidone (Supplementary Figure 1, available online).
Reasons for first treatment failure
In all 3 subgroup analyses, arrest/incarceration (range, 20.0%–31.1%) and psychiatric hospitalization (range, 8.0%–15.3%) were the most common reasons for first treatment failure (Table 3). No suicides were reported.
Table 3 Reasons for first treatment failure (intent-to-treat population)
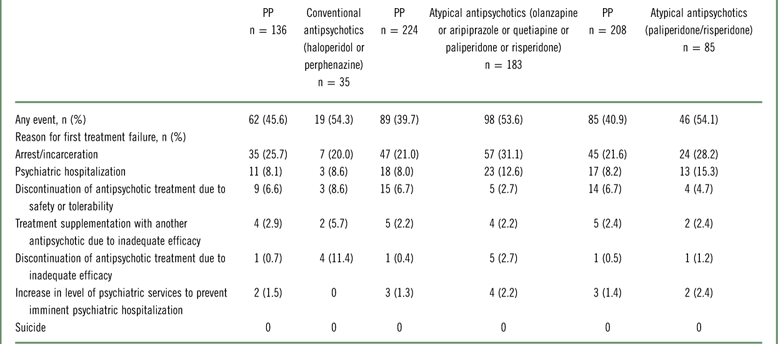
Abbreviation: PP, paliperidone palmitate.
Safety
Treatment-emergent AEs (TEAEs) were reported in 91.4%, 77.6%, and 77.6% of subjects in the conventional antipsychotic, atypical antipsychotic, and paliperidone plus risperidone groups, respectively (Table 4). The frequency of TEAEs in the once-monthly PP groups ranged from 85.7% to 87.0%.
Table 4 Summary of treatment-emergent adverse events in ≥5% of subjects in any group by preferred termFootnote a (intent-to-treat analysis)
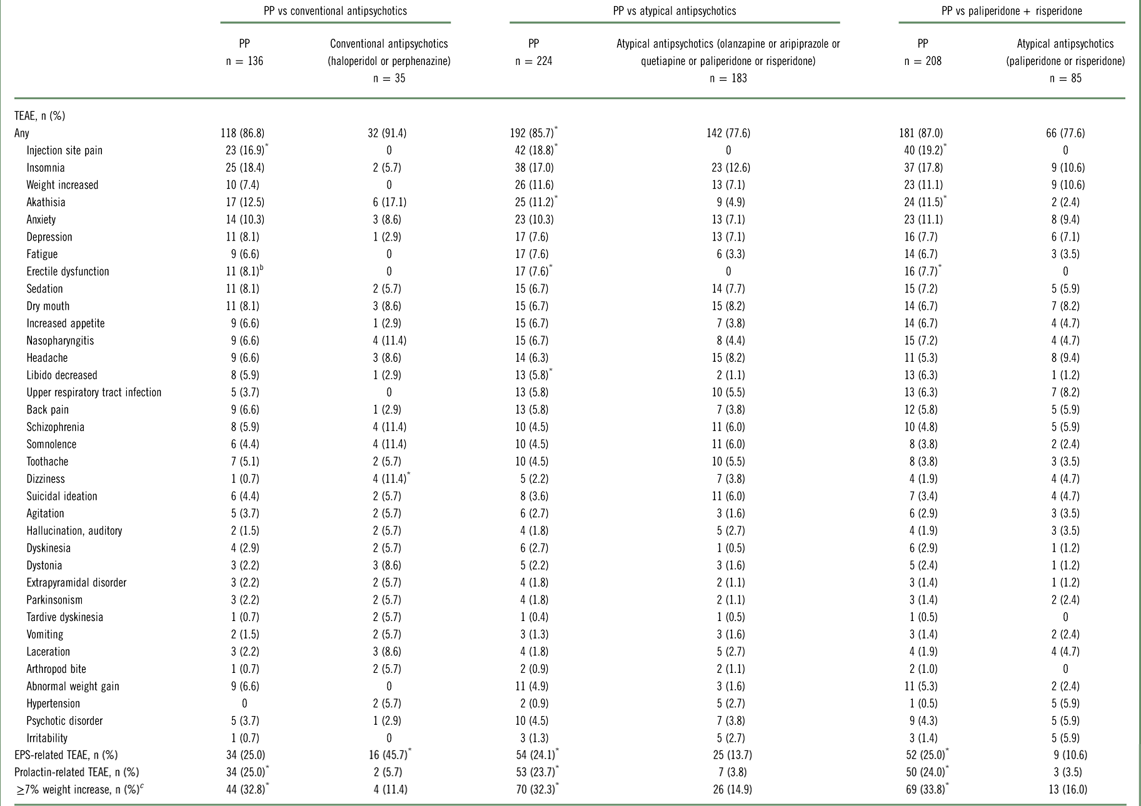
Abbreviations: EPS, extrapyramidal symptom; PP, paliperidone palmitate; TEAE, treatment-emergent adverse event.
a Preferred terms of adverse events were based on version 12.0 of the Medical Dictionary for Regulatory Activities (MedDRA).
b Percentages based on male participants only were 11/118 (9.3%) for conventional antipsychotics, 17/191 (8.9%) for atypical antipsychotics, and 16/176 (9.1%) for paliperidone/risperidone.
c Month 15, last observation carried forward.
*P<.05, Pearson’s chi-square test.
Serious AEs occurred in 20.0%, 21.9%, and 24.7% of subjects in the conventional antipsychotic, atypical antipsychotic, and paliperidone plus risperidone groups, respectively. The incidence of serious AEs ranged from 17.4% to 19.9% in the once-monthly PP groups. TEAEs leading to study drug discontinuation occurred in 22.9%, 4.9%, and 7.1% of subjects in the conventional antipsychotic, atypical antipsychotic, and paliperidone/risperidone groups, respectively, and ranged from 11.5% to 12.1% in the once-monthly PP groups.
EPS-related TEAEs were reported more frequently by subjects in the conventional antipsychotic group (45.7%) compared with those in the atypical antipsychotic (13.7%), paliperidone plus risperidone (10.6%), or once-monthly PP (range, 24.1%–25.0%) groups (Table 4). Prolactin-related TEAEs were more commonly reported in the once-monthly PP groups (range, 23.7%–25.0%) than in the oral antipsychotic groups (range, 3.5%–5.7%). Alphs et al Reference Alphs, Benson, Cheshire-Kenny, Lindenmayer, Mao and Rodriguez 29 reported on an increased incidence of prolactin-related TEAEs with once-monthly PP vs oral antipsychotics that was apparent in both males and females. A≥7% increase in body weight at month 15 (last observation carried forward) was observed in approximately one-third of subjects who received once-monthly PP (range, 32.3%–33.8%) compared with 11.4%, 14.9%, and 16.0% of subjects in the conventional, atypical, and paliperidone/risperidone groups. One death occurred in the PP group and was considered by the investigator as unlikely related to study drug.
Discussion
These exploratory analyses from the PRIDE study show that the risk of treatment failure was 34% and 41% higher with oral conventional antipsychotics and oral atypical antipsychotics, respectively, when compared with PP. Although subgroup comparison with oral conventional antipsychotics did not reach statistical significance, which was likely due to small and underpowered subsamples, the effectiveness results were consistent with the primary analysis of PRIDEReference Alphs, Benson, Cheshire-Kenny, Lindenmayer, Mao and Rodriguez 29 and support the premise that improvements observed with PP are consistent across classes of treatments (oral conventional antipsychotics and oral atypical antipsychotics). Our exploratory analyses also evaluated the effect of delivery method on outcome. Compared with PP, the risk for treatment failure was 39% higher with oral delivery of similar or identical molecules (paliperidone/risperidone), suggesting that there may be differences in the effectiveness between the 2 formulations. The primary driver of this difference may be attributable to the longer half-life of PP, which provides a longer duration of continuous effective exposure than the oral formulation.Reference Chue and Chue 33 Unlike oral medications, LAI formulations provide the physician with certain knowledge of adherence and with greater opportunity to correct nonadherence before marked declines in plasma levels occur.Reference Kane, Kishimoto and Correll 34
The overall higher incidence of AEs observed in PRIDE was likely due to its somewhat unique study design. In studies where patients are stabilized before randomization, patients may drop out due to AEs before randomization occurs. Patients in PRIDE were followed from the first dose; therefore, the duration of AE reporting was longer compared with that of standard trial designs. As expected, the greater adherence and medication exposure associated with an LAI contribute to differing AE profiles compared to those for oral antipsychotics. Differential AE profiles include higher rates of injection site pain, prolactin-related AEs, and weight gain associated with PP, and higher rates of EPS-related AEs associated with oral conventional antipsychotics.Reference Peuskens, Pani, Detraux and De 35 – Reference Citrome 37 These findings support the knowledge that conventional oral antipsychotics have clinically relevant EPS-related AEs that may limit their clinical utility. It must be considered, however, that AE reporting rates may reflect true differences, greater adherence with PP, and/or biases introduced by the deselection process for this study, which would favor patients who deselected oral antipsychotics that had led to relevant AEs in the past. For these reasons, the unique study design of PRIDE likely impacted AE profiles. For example, in the PROSIPAL study, which evaluated 24-month outcomes of patients treated with PP compared with oral antipsychotics (aripiprazole, quetiapine, olanzapine, paliperidone extended release, risperidone, or haloperidol) in patients with recently diagnosed schizophrenia, rates of prolactin AEs were similar at 6.3% and 5.0%, respectively.Reference Schreiner, Aadamsoo and Altamura 38 Similarly, a post hoc analysis that compared data from a PP trial (median of 170 days of exposure) and an oral paliperidone extended-release trial (median of 45 days of exposure) showed comparable rates of TEAEs of interest, including prolactin-related AEs (2.1% and 2.9%, respectively).Reference Markowitz, Fu, Levitan, Gopal, Turkoz and Alphs 39 Owing to differences in trial design, caution must be used when comparing PRIDE to other studies.
Several limitations should be considered when interpreting these data. First, although findings from PRIDE are more reflective of real-world outcomes than trials with extensive exclusion and inclusion criteria, these data cannot be generalized to all patients with schizophrenia. Indeed, subpopulations may have more or less effect. Second, subgroup analyses had lower N values than the overall analysis, and caution should be exercised when interpreting findings in subgroups. Third, despite randomization, the preselection of suitable oral antipsychotics before randomization could have introduced selection bias in favor of the oral antipsychotic group because only the oral medication(s) selected could be randomly assigned if patients were not randomly assigned to the PP arm. The deselection of specific oral antipsychotics as well as low compliance with oral antipsychotics may have biased the safety results, masking tolerability issues associated with any individual oral antipsychotic. Fourth, due to the nature of exploratory analyses, multiplicity adjustments were not performed; hence, the overall type I error would be greater than 5%, the nominal level used for each individual test.
Collectively, these data suggest that PP confers effectiveness advantages over oral antipsychotic therapies. However, in the current study design, these advantages seemed to be associated with greater patient-reported AEs (except for lower EPS-related AEs vs the conventional oral antipsychotic group). Because PRIDE included patients normally excluded from clinical trials (eg, those with a history of contact with the criminal justice system), these findings are particularly noteworthy, as they reflect patients with real-world characteristics who were treated in real-world paradigms using real-world outcomes. Given the unique study design of PRIDE, additional analytic approaches may be explored, such as covariate analyses that adjust for baseline characteristics and time-dependent variables.
Conclusions
Exploratory analyses of PRIDE confirm findings from the primary analysis and preliminarily suggest that the advantages of once-monthly PP in effectiveness apply across all antipsychotic classes (ie, between once-monthly PP and conventional oral antipsychotics and between once-monthly PP and atypical oral antipsychotics). As expected, differential AE profiles were observed between the conventional oral antipsychotics, atypical oral antipsychotics, and once-monthly PP groups. Given the varied safety profiles among these agents, pooled data and the deselection process could have masked tolerability issues associated with any one oral agent. These findings provide insight into the differential effectiveness and side-effect profiles of the once-monthly PP versus daily oral conventional antipsychotics and daily oral atypical antipsychotics; however, larger groups of patients are required to draw more definitive conclusions.
Disclosures
E. Kim, H. L. Starr, and L. Alphs are employees of Janssen Scientific Affairs, LLC, and are Johnson & Johnson stockholders. L. Mao is an employee of Janssen Research and Development, LLC, and is a Johnson & Johnson stockholder. C. U. Correll has been a consultant and/or advisor to or has received honoraria from AbbVie, Acadia, Actavis, Alkermes, Eli Lilly, Forum, Genentech, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, Lundbeck, MedAvante, Medscape, Otsuka, Pfizer, ProPhase, Reviva, Roche, Sunovion, Supernus, Takeda, and Teva. He has received grant support from Bristol-Myers Squibb, Otsuka, Lundbeck, and Takeda. C. U. Correll reports personal fees from Janssen/J&J, AbbVie, Acadia, Actavis, Alkermes, Eli Lilly, Genentech, Gerson Lehrman Group, IntraCellular Therapies, Lundbeck, MedAvante, Medscape, Otsuka, Pfizer, ProPhase, Reviva, Roche, Sunovion, Supernus, Takeda, and Teva, outside the submitted work.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/10.1017/S1092852916000444








