Case description
A 15-month-old male weighing 6.5 kg was referred for shortness of breath and tachypnoea. History revealed that patient was diagnosed with Kabuki syndrome. Patient was found to be tachycardic and tachypnoeic at examination. On the chest X-ray, pulmonary congestion and enlarged left atrium were evident. Electrocardiogram showed P-mitrale pattern, suggestive of left atrial enlargement. Echocardiography showed normal left and right ventricular systolic function. Left ventricle, left ventricular outflow tract, aortic valve, and ascending aorta were normal in size (z-scores of around −1). Mitral valve was stenotic with fused papillary muscles directly attached to mitral valve leaflets without distinct chordal attachments. Mean mitral inflow gradient was 17 mmHg with minimal mitral regurgitation. Left atrium was severely enlarged and right ventricle systolic pressure was measured as 65–70 mmHg. There was no other morphologic abnormality (Fig. 1). Patient was hospitalised and put on inotropes and diuretics. Because of the accompanying lower respiratory tract infection, IV antibiotic therapy was also initiated. He was discussed in paediatric heart team meeting and decision was made for mitral valve surgery.
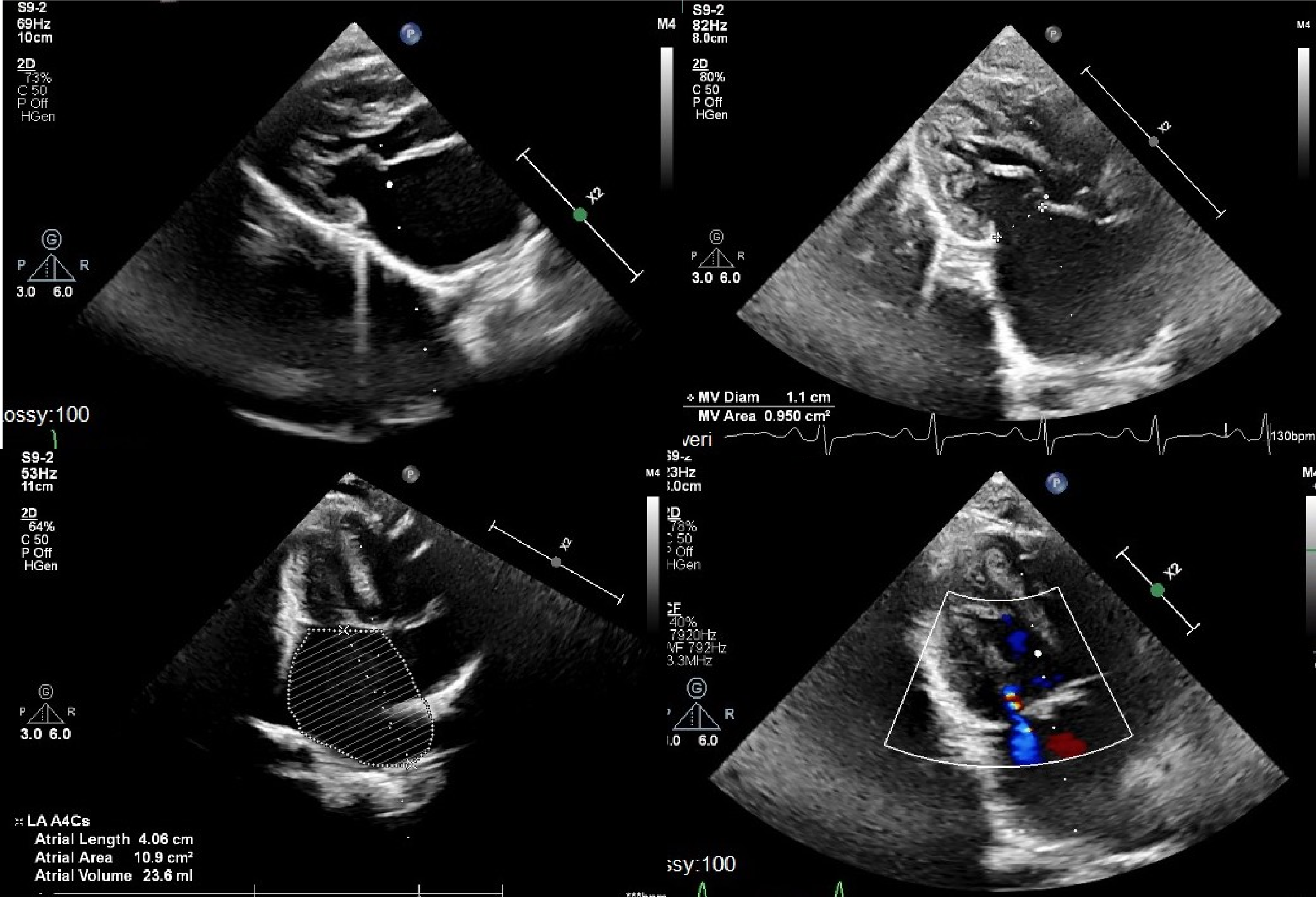
Figure 1. Pre-operative echocardiography images showing left atrial dilatation, severe multilevel congenital mitral stenosis, and hypoplastic mitral valve annulus.
Having cleared the lower respiratory tract infection and upon approval by the infectious diseases team, patient was brought to the operating room. A median sternotomy was done. Left atrium was enlarged and pulmonary veins were dilated. Cardiopulmonary bypass was instituted with standard aortic and bi-caval cannulations. Following the cross-clamp and cardioplegic arrest, left atrium was opened through interatrial groove. Anatomy was inspected. Multilevel complex stenosis of the mitral valve was seen. Annulus and orifice were extremely stenotic. A hypoplastic annulus free of supravalvar involvement was the first finding. Both leaflets were extremely thickened and rolled in addition to being extremely dysplastic. Even a 4 mm dilator was barely able to pass through the extremely narrow valvar opening. Additionally, the papillary muscles were not evenly distributed; one of them was extremely hypoplastic, and they were somewhat close together. The one that predominated was long, angled toward the annulus, bridged by fibrous tissue to the mitral leaflet’s free edge, and situated high in the left ventricle. There were no evident interchordal spaces, and the chordae tendinae were thickened, shortened, and packed into the subvalvar area, which was mostly attached to the dominant papillary muscle (Fig. 2). The extreme morphologic structure made it impossible to perform a decent and long-lasting repair, so the decision was made to replace the valve. Mitral valve and its apparatus were completely excised, leaving adequate rim for replacement sutures. Even after complete excision of the valve, 17 mm Hegar dilator was barely passing through the annulus. Thus, 15 mm mechanical aortic valve was implanted with supra-annular technique in upside-down fashion to prevent heart block, circumflex coronary artery compression, and pulmonary vein stenosis risks. Having implanted the valve, left atriotomy was closed and patient was weaned off cardiopulmonary bypass uneventfully with low dose inotrope. Post-bypass epicardial echocardiography showed good biventricular function, no mitral paravalvular regurgitation, or prosthetic gradient. The aortic cross-clamp and bypass times were 75 and 93 minutes, respectively. Post-operative course was uneventful. The patient was extubated on the day of operation, and drains and wires were removed on postoperative day 3. Pre-discharge echocardiogram showed normal systolic function without any prosthetic valve problem. Subsequently, he was discharged to home on postoperative day 6 with adequate international normalized ratio (INR) follow-up scheme. He is still doing clinically well 25 months after the operation with a mean INR value of 2.6.
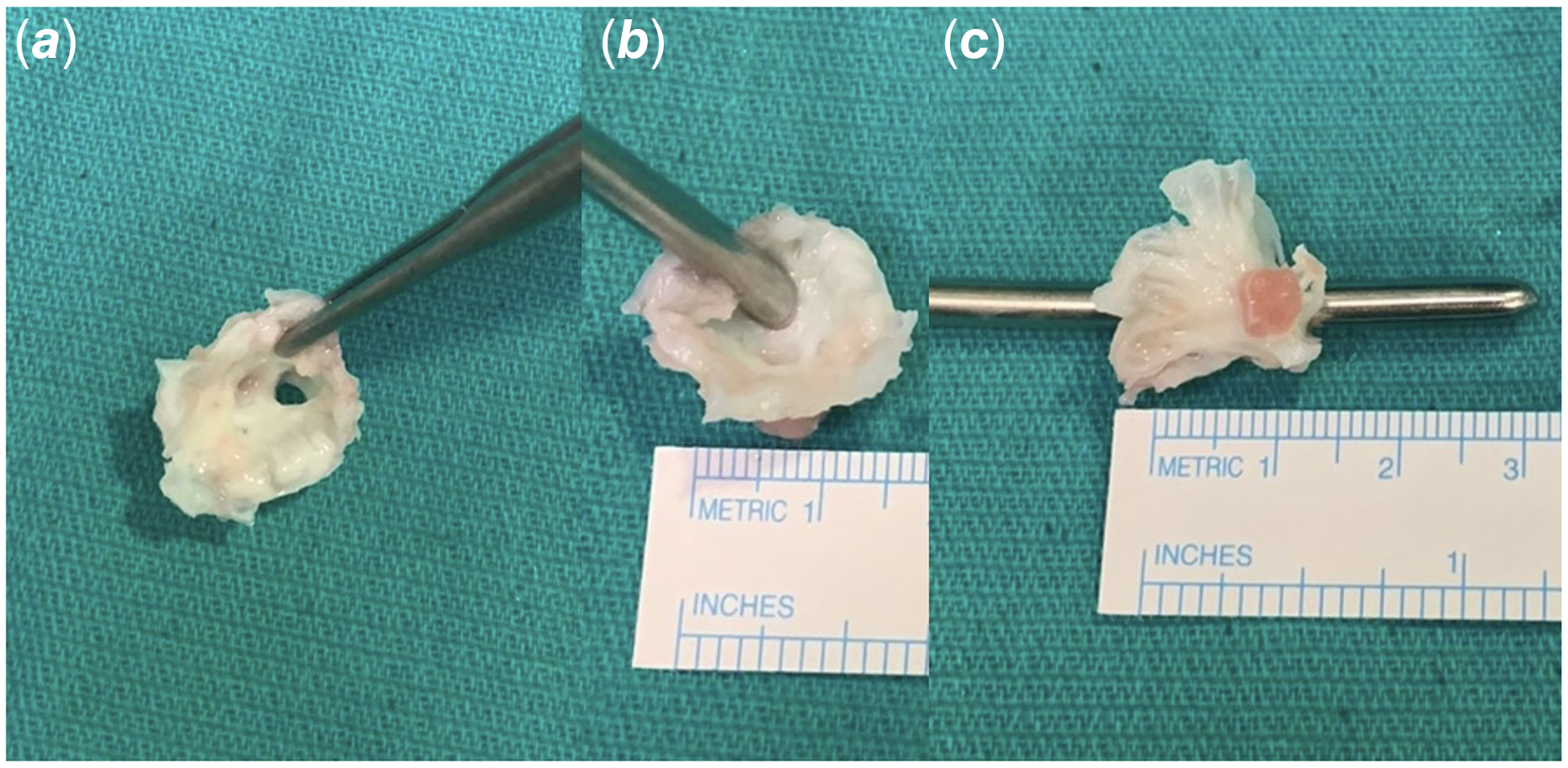
Figure 2. Intra-operative resected mitral valve. Severely dysplastic mitral valve ( a ); 4 mm Hegar dilator was barely passing through the valvar orifice ( b ); Severely dysplastic mitral valve with subvalvar apparatus showing one dominant and one another hypoplastic papillary muscles ( c ).
Discussion
Congenital malformations of the mitral valve were identified in approximately 0.5% of the 13,400 subjects in an echocardiographic study. Reference Moore, Adatia and Spevak1,Reference Maskatia2 Isolated congenital mitral stenosis is a quite rare entity. Congenital mitral stenosis is usually associated with other cardiac anomalies such as Shone’s complex, subvalvular aortic stenosis, and aortic coarctation. Reference Remenyi and Gentles3,Reference Séguéla, Houyel and Acar4 Typical congenital mitral stenosis is characterised by thickened and folded leaflets, chordae tendineae that are shortened and lacking interchordal spaces, and papillary muscles that are underdeveloped and closely spaced. Thus, basic classifications like subvalvar, valvar, or supravalvar stenosis represent more benchmarks along a continuous spectrum as opposed to completely separated groups. The preoperative evaluation of the anatomical substrate is crucial for the successful management of congenital mitral stenosis. Echocardiographic studies of congenital mitral valve disorders are now typically performed level by level. Thus, the mitral valve, including the valvar leaflets, tensor apparatus, and papillary muscles, should be evaluated as an entire complex. Reference Moore, Adatia and Spevak1–Reference Banerjee, Kohl and Silverman5 This approach leads to a final diagnosis from the morphologic and physiologic perspectives. Varying effects of these lesions may be observed on valve function. The severity of the disease is influenced by various factors, including the degree of obstruction, the presence of regurgitation, the existence and severity of associated pulmonary hypertension, and the existence of associated heart and non-cardiac malformations. When indicated, surgical repair provides good long-term results.
Kabuki syndrome is a genetically heterogeneous disorder distinguished by skeletal anomalies, congenital heart defects, developmental delay, anorectal anomalies, persistence of fetal fingertip pads, developmental delay, growth defect with feeding difficulties, anorectal anomalies, renal malformations, anorectal anomalies, and the presence of distinct facial features such as sparse eyebrows and long palpebral fissures, large everted ears, eversion of the lateral third of the lower eyelids, and a pillowed lower lip. Reference Digilio, Gnazzo and Lepri6 CHDs are quite common in Kabuki syndrome with almost all being left-sided cardiac anomalies like aortic coarctation, hypoplastic left heart syndrome, Shone syndrome, aortic valve stenosis, and mitral valve stenosis. Isolated congenital mitral stenosis in Kabuki syndrome is also rare, with being reported only in two cases before. Reference Digilio, Gnazzo and Lepri6,Reference Yoon, Ahn and Kwon7
In our patient when the mitral valve was examined intraoperatively, it was found to be not appropriate for satisfactory repair as the valve was showing severe stenotic features in multilevel fashion. First, the annulus was hypoplastic with no supravalvar involvement. Secondly, both leaflets were severely dysplastic, thickened, and rolled. Valvar orifice was so small even 4 mm dilator was barely passing through. Third, there were two asymmetric papillary muscles, one being severely hypoplastic, and they were closely spaced. The dominant one was elongated, displaced toward the annulus, connected to the free edge of the mitral leaflet by a bridge of fibrous tissue, and highly positioned within the left ventricle. It had a wide base and was attached to the lateral side of the left ventricle. Subvalvar area was crowded with chordae tendinae were shortened and thickened with the absence of interchordal spaces and predominantly connected to the dominant papillary muscle.
With these morphologic features, we opted for valve replacement as repair was not feasible. The surgical outcomes for congenital mitral stenosis exhibit considerable variability, with a strong dependence on factors such as the experience of the institution, the age and weight of the patient, the extent of annular hypoplasia, the presence of associated left-sided obstructive lesions, and the severity of pulmonary hypertension. In paediatric patients, the preference lies with valve repair over valve replacement. Mitral valve replacement is associated with significant risk, as evidenced by operative mortality rates ranging from 0 to 36%, despite the notable progress made in surgical methods and the availability of several valve options. However, when repair is not anatomically feasible, valve replacement is performed as the last recourse due to the fact that suboptimal primary repair is a significant predictor of reoperation and further morbidity and mortality risks. Reference Henaine, Roubertie, Vergnat and Ninet8,Reference Alghamdi, Yadava and Van Arsdell9
Several techniques are described for mitral valve replacement in small children with small annulus. Unlike aortic valve replacement, options for annular enlargement are limited. Subaortic obstruction may result from an attempt to enlarge the prosthesis during MVR; this course of action should be avoided. Supra-annular replacement of the valve in children with a small annulus has favourable operative survival and low heart block rate. Reference Henaine, Roubertie, Vergnat and Ninet8–Reference Overman, Moga, Stephens, Dearani and MacIver10 Considering the small annulus of our patient, we also decided to implant the mechanical valve in supra-annular fashion. Post-operative course was uneventful without any rhythm problems and he has been doing well clinically during the 10-months follow-up with regular INR checks.
Although it is widely recognised that syndromic patients often have a poorer prognosis compared to those without syndromes, we maintain the belief that attaining a competent valve with replacement would be beneficial in long-term without any additional procedures while being closely monitored.
Financial support
None.
Competing interests
None.
Ethical standard
Informed consent from the patient’s parents was obtained.





