In recent decades, antibodies targeting intracellular, cell surface and synaptic neural antigens have emerged as biomarkers that aid in the diagnosis of autoimmune encephalitis. Reference Graus, Titulaer and Balu1 Many of these neural antibodies are detected using brain tissue indirect immunofluorescence (TIIF) to identify characteristic staining patterns, followed by a second assay to confirm antibody specificity. Detection of antibodies against intracellular neural antigens, including well-characterised paraneoplastic or “onconeural” antibodies (anti-Hu, Yo, Ri, amphiphysin, CV2/collapsin response mediator protein 5 (CRMP5) and Ma2), typically does not require that the target antigen be in its native conformation, permitting the use of Western blot/line immunoblot (WB/LIB) as a second confirmatory assay alongside TIIF. Reference Waters, Pettingill and Lang2 In contrast, detection of antibodies against extracellular cell surface/synaptic antigens typically requires that critical epitopes remain in their native conformation, leading to the use of transfected cell-based assays (CBAs) expressing the antigen of interest on their surface as a second confirmatory assay alongside TIIF. Reference Waters, Pettingill and Lang2
Testing for neural antibodies has historically been performed by a small number of reference laboratories with dedicated expertise in this area. In recent years, however, the advent of commercialised assays has afforded laboratories with an interest in autoimmune neurology the opportunity to offer neural antibody testing, which has the advantage of reduced cost and improved turnaround times compared to send out testing. Due to relative ease of test implementation and interpretation of WB/LIB and CBA compared to TIIF, some laboratories have opted to offer stand-alone commercial assays that were initially described as confirmatory (i.e. WB/LIB, CBA) without TIIF for neural antibody detection. Studies validating this approach to neural antibody testing in clinical practice, however, are lacking, and indeterminate results of uncertain clinical significance have been reported. Reference McCracken, Zhang and Greene3 We previously demonstrated that the positive predictive value of onconeural antibody testing for paraneoplastic neurological syndromes (PNS) to be only 39% when reporting commercial LIB positivity alone without TIIF Reference Budhram, Nicolle and Yang4 . Our findings highlighted that while commercial LIB is a useful confirmatory assay when TIIF staining is concerning for a particular onconeural antibody, its use as a stand-alone assay may lead to a high number of false-positive results. In order to offer neural antibody testing for autoimmune encephalitis, we introduced a panel of CBAs for the detection of neural antibodies against extracellular cell surface/synaptic antigens (N-methyl-D-aspartate receptor (NMDAR), leucine-rich glioma-inactivated protein 1 (LGI1), contactin-associated protein-like 2 (CASPR2), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), γ-aminobutyric acid type B receptor (GABABR), dipeptidyl-peptidase-like protein-6 (DPPX), IgLON family member 5 (IgLON5)) as well as the intracellular synaptic antigen glutamic acid decarboxylase-65 (GAD65), performed in parallel with TIIF. Similar to our examination of onconeural antibody testing, we reviewed the results of our autoimmune encephalitis panel after its first year of implementation with the primary aim of identifying possible false-positive results for quality assurance. As per our institutional research ethics board, quality assurance and quality improvement studies do not fall within the scope of institutional ethical review under Article 2.5 of the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2), but all Pathology and Laboratory Medicine quality assurance/quality improvement studies are still reviewed departmentally to address any ethical issues that may arise.
Between March 2019 and March 2020, we received serum and/or CSF samples from 373 patients for autoimmune encephalitis antibody testing. All samples were tested in parallel by rodent hippocampus and cerebellum TIIF (EUROIMMUN, Order No. FA 111 m-1005-3) as well as fixed CBA (EUROIMMUN, Order No. FA112d-1005-6, FA112d-1010-6, FA 1022-1005-50, FA 1151-1005-50) for eight neural antibodies (anti-NMDAR, LGI1, CASPR2, AMPAR, GABA(B)R, DPPX, IgLON5 and GAD65) using the manufacturer’s instructions. Testing was performed at a 1:10 dilution for serum and undiluted for CSF. Samples were processed using the automated immunoassay analyzer (IF Sprinter, EUROIMMUN). A weakly positive or positive CBA, with or without corresponding TIIF positivity, was required for a positive result to be reported (automated microscopy by EUROPattern, EUROIMMUN). TIIF and CBA were each reported as negative, weakly positive or positive based on independent interpretation by two readers with experience in indirect immunofluorescence (P.E. and L.Y.). In cases with uncertainty regarding positive staining, images taken by automated microscopy were submitted to the assay manufacturer (EUROIMMUN) for additional review with discussion to achieve consensus. “Weakly positive” referred to staining that was faint, but of sufficient intensity above the background to be possibly indicative of a positive result (see Figure 1); the distinction between “weakly positive” and “positive”, while subjective, reflects potential challenges of indirect immunofluorescence interpretation in clinical practice. A clinical questionnaire containing pertinent clinical information was requested with each patient sample, to allow for clinical–serological correlation and identification of potential false-positive results as a quality assurance measure (see Supplementary document).
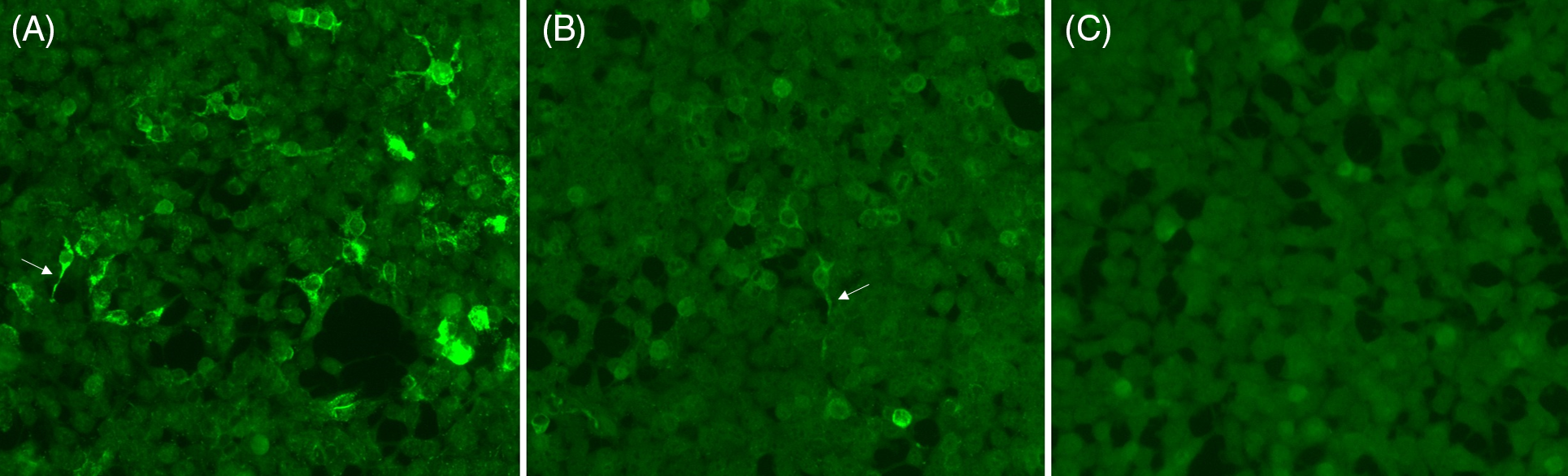
Figure 1: Representative staining of positive, weakly positive and negative neural antibody testing by CBA. Positive staining (A), weak positive staining (B) and negative staining (C) for N-methyl-D-aspartate receptor (NMDAR) antibodies are shown; positive (A) and weak positive (B) samples show staining of cytoplasmic extensions typical of anti-NMDAR (arrows).
Over this 1-year period, 20/373 patients (5.4%) had a positive neural antibody reported (see Figure 2). Thirteen out of the 20 had only serum submitted, 1/20 had only CSF submitted and 6/20 had both serum and CSF submitted. Positive results consisted of anti-CASPR2 (6/20), NMDAR (5/20), LGI1 (4/20), GAD65 (4/20) and GABA(B)R (1/20). No sample tested positive for more than one analyte. Clinical information provided for these 20 patients to aid in clinical–serological correlation as a quality assurance measure was reviewed by a neurology resident and a neurologist with subspecialty training in Autoimmune Neurology (A.M. and A.B.). Those with a compatible clinical phenotype based on the available literature and no more likely alternative diagnosis were classified as true positives, Reference Gadoth, Pittock and Dubey5–Reference Höftberger, Titulaer and Sabater8 while all other patients were flagged as possible false positives (see Table 1).

Figure 2: Flow diagram depicting classification of patients with true-positive versus false-positive autoimmune encephalitis antibody testing.
Table 1: Possible false-positive results in patients undergoing neural antibody testing for suspected autoimmune encephalitis
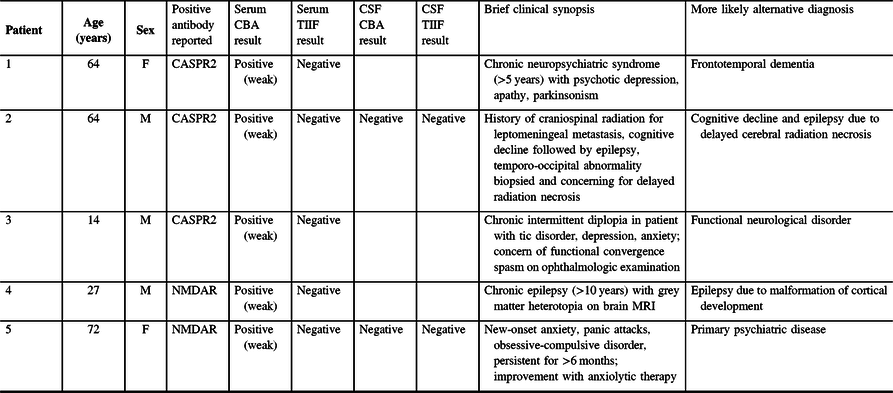
CASPR2 = contactin-associated protein-like 2; CBA = cell-based assay; NMDAR = N-methyl-D-aspartate receptor; TIIF = tissue indirect immunofluorescence.
Amongst antibody-positive patients, all patients with anti-LGI1, GAD65 or GABA(B)R positivity were classified as true positives. All four patients with anti-LGI1 positivity had new-onset focal seizures, three of whom had faciobrachial dystonic seizures. Three out of the four were positive in serum by CBA (no CSF testing was performed), and 1/4 was positive in CSF by CBA (no serum testing was performed). Only 2/4 patients were positive for anti-LGI by TIIF (one serum, one CSF). Amongst four patients with anti-GAD65 positivity, two had chronic temporal lobe epilepsy, and two had a clinical/radiographic presentation concerning for autoimmune limbic encephalitis. All four patients were positive for anti-GAD65 in serum by CBA and TIIF. Two out of the four underwent CSF testing; both were positive for anti-GAD65 by CBA in CSF, and 1/2 was positive for anti-GAD65 by TIIF in CSF. The patient with anti-GABA(B)R positivity had a clinical/radiographic presentation concerning for autoimmune limbic encephalitis. Serum was positive for anti-GABA(B)R by CBA but not TIIF, while CSF was positive for anti-GABA(B)R by CBA and TIIF.
In contrast, only 3/6 patients with anti-CASPR2 positivity were classified as true positives; 2/3 had new-onset temporal lobe epilepsy, and 1/3 had cognitive decline with neuropathic pain. All three patients were positive for anti-CASPR2 in serum by CBA (2/3 with weakly positive staining); none were positive by TIIF in serum and none had CSF testing performed. The remaining 3/6 patients with anti-CASPR2 positivity (all serum samples, all only weakly positive by CBA) were flagged as possible false-positive results (see Table 1). None were positive for anti-CASPR2 by TIIF in serum; 1/3 underwent CSF testing that was negative for anti-CASPR2 by CBA and TIIF. Similarly, amongst patients with anti-NMDAR positivity, only 3/5 were classified as true positives; all three had a subacute neuropsychiatric syndrome with psychosis, dysautonomia, dyskinesias, seizures and/or memory impairment. Two out of the three patients were positive for anti-NMDAR by CBA and TIIF in serum (CSF testing was not performed in either); 1/3 was negative for anti-NMDAR in serum but positive in CSF by CBA and TIIF. The remaining 2/5 patients with anti-NMDAR positivity (both serum samples, both only weakly positive by CBA) were flagged as possible false-positive results (see Table 1). Neither were positive for anti-NMDAR by TIIF in serum; 1/2 underwent CSF testing that was negative for anti-NMDAR by both CBA and TIIF.
Our findings provide several valuable insights into neural antibody test implementation for autoimmune encephalitis. First, clinical sensitivity for detection of neural antibodies studied herein seems overall higher by CBA than by TIIF, as evidenced by true-positive samples by CBA that were negative by TIIF. This highlights the value of CBA when implementing neural antibody testing in suspected autoimmune encephalitis. However, the higher clinical sensitivity of CBA may come at some expense to clinical specificity, most notably for serum anti-CASPR2 and NMDAR. We found that weak serum positivity for anti-CASPR2 by CBA lacked clinical relevance in some cases, which is consistent with previous reports. Reference Bien, Mirzadjanova and Baumgartner9 For this reason, the clinical correlation of a weakly positive serum anti-CASPR2 by CBA is paramount, and in patients with an atypical presentation for anti-CASPR2 neurological autoimmunity, an alternative diagnosis should be sought. As per the manufacturer’s recommendation (released after completion of this evaluation), CBA testing for anti-CASPR2 at a higher serum dilution than 1:10 (e.g. 1:100) to determine if there is persistent positivity may help exclude clinically irrelevant weak positive staining; we plan to evaluate this moving forward. Isolated weak serum positivity for anti-NMDAR by CBA should also be interpreted with caution, as noted previously. Reference Titulaer, Kayser and Dalmau10 In such cases, we recommend CSF evaluation because CSF testing has higher clinical sensitivity and specificity for anti-NMDAR encephalitis. Reference Gresa-Arribas, Titulaer and Torrents11,Reference Brooks, Yarbrough, Bucelli and Day12 No other CBAs raised concern for false-positive results in this 1-year period, although small sample sizes preclude broad generalisation of this finding. Interestingly, all four patients with serum anti-GAD65 positivity by CBA were classified as true positives. Historically, the clinical relevance of serum anti-GAD65 positivity in suspected neurological autoimmunity has been correlated with high anti-GAD65 levels by quantitative assays such as radioimmunoassay or enzyme-linked immunosorbent assay. Reference Graus, Saiz and Dalmau7 Newer commercial qualitative assays such as CBA usually indicate high levels of anti-GAD65 if positive, in keeping with our findings of a clinically relevant neurological syndrome amongst all four patients with anti-GAD65 positivity by this methodology. Reference Graus, Saiz and Dalmau7 Across all neural antibodies, no patient with a possible false-positive serum result by CBA had corresponding serum positivity by TIIF, or CSF positivity by CBA or TIIF; the presence of either is thus likely indicative of a true-positive serum CBA result, and submission of both serum and CSF to maximise diagnostic accuracy of neural antibody testing is generally recommended.
This quality assurance evaluation of neural antibody testing for suspected autoimmune encephalitis by CBA aligns with the published experiences of leading international laboratories, Reference Graus, Saiz and Dalmau7,Reference Bien, Mirzadjanova and Baumgartner9,Reference Titulaer, Kayser and Dalmau10 indicating appropriate neural antibody test implementation and interpretation locally. Limitations include the retrospective nature of this single-centre experience performed as a quality assurance measure, lack of extensive clinical information or comparison assays as this is not part of our routine laboratory reporting practice, and the relatively small number of positive results. Prospective, multicentre studies are needed to fully delineate the clinical and serological findings of patients with suspected autoimmune encephalitis in Canada. Nonetheless, our findings have clear clinical relevance to Canadian neurologists who order this testing, and can aid laboratories in optimising the diagnostic accuracy of neural antibody testing offered locally.
Acknowledgements
The authors would like to acknowledge Dominik Jaeger and Erik Lattwein for their review of the manuscript.
Funding
The authors acknowledge the generous financial assistance by EUROIMMUN Medical Diagnostics in the initial implementation of neural antibody testing for autoimmune encephalitis at the London Health Sciences Centre, which was provided as part of a quality assurance initiative to improve neural antibody testing in patients with suspected autoimmune encephalitis. No funding was provided for this submission.
Conflict of Interest
Sean McFadden is a EUROIMMUN Medical Diagnostics Employee, but was not involved in the clinical-serological correlation of neural antibody results. The other authors have no disclosures to report.
Statement of Authorship
AB is a Neurologist and Assistant Professor of Neurology who analysed the data and drafted the manuscript. AM is a Neurology Resident who analysed the data and reviewed the manuscript for intellectual content. SM is a EUROIMMUN Medical Diagnostics Employee who reviewed the manuscript for intellectual content. PE is a Senior Laboratory Technologist in the Clinical Immunology Laboratory who analysed the data and reviewed the manuscript for intellectual content. VB is a Clinical Chemist, Associate Professor of Pathology and Laboratory Medicine, and the Division Head of Biochemistry & Immunology who reviewed the manuscript for intellectual content. LY is a Clinical Biochemist, Assistant Professor of Pathology and Laboratory Medicine, and the Section Head of the Clinical Immunology Laboratory who analysed the data and reviewed the manuscript for intellectual content.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2021.23.





