Hypovitaminosis D is highly prevalent in India despite adequate sunshine. Factors responsible for this include inadequate exposure to sunshine, atmospheric pollution and skin pigmentation. Serious consequences of vitamin D deficiency include cardiac failure and hypocalcaemic seizures( Reference Mehrotra, Marwaha and Aneja 1 ). Transplacental transfer and breast milk concentration of vitamin D are low in mothers with poor vitamin D status during pregnancy and lactation( Reference Jain, Gupta and Kalaivani 2 – Reference Misra, Pacaud and Petryk 4 ). Studies from India have shown 84–96 % of mothers and infants at birth and at 3 months to have serum 25-hydroxyvitamin D (25(OH)D)<20 ng/ml (50 nmol/l), with winter mean 25(OH)D in women being as low as 5·9 ng/ml (14·75 nmol/l)( Reference Jain, Gupta and Kalaivani 2 , Reference Sachan, Gupta and Das 5 ).
High-dose (160µg or 6400 IU/d) vitamin D3 administered to the lactating mother was found to safely and significantly improve maternal 25(OH)D and mean milk antirachitic activity( Reference Hollis, Wagner and Howard 6 ). Ala-Houhala et al.( Reference Ala-Houhala, Koskinen and Terho 7 ) showed that a regimen of 50 (but not 25) μg/d to the mother improved the nursing infant’s serum 25(OH)D significantly. In the only study from India on postnatal supplementation of term infants, Kumar et al.( Reference Kumar, Sachdev and Chellani 8 ) did not find any differences between the placebo and supplemented groups (receiving 35µg vitamin D3/week) in mortality or hospital admissions (their primary outcomes) or referral to the outpatient clinic for moderate morbidity, although serum 25(OH)D was higher in the treated group. Current international recommendations mention 10µg/d as routine supplementation for breast-feeding infants( Reference Wagner and Greer 3 , Reference Misra, Pacaud and Petryk 4 , Reference Ross, Manson and Abrams 9 ).
However, no study from tropical countries has documented the result of specific sun exposure advice for infants. Furthermore, maternal supplementation must be explored as a means of supplementing the infant and mother at the same time. We undertook a study to document whether important biological correlates such as infant parathyroid hormone (PTH), hypocalcaemia, growth and propensity to infection would be favourably altered by vitamin D supplementation to the infant. We hypothesised that vitamin D3 supplementation to the lactating mother at a dose of 3000µg/month (which would be similar to a daily dose of 100µg) would improve the vitamin D status in both lactating women and their breast-feeding infants and that this form of supplementation would be comparable to oral vitamin D3 supplementation of 10µg/d to the infant and superior to placebo.
Methods
Study design and subjects
The study was carried out between September 2012 and June 2014 at the King George Medical University, and Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow (latitude 26°N). In northern India, the monsoon season is concluding by mid September, December and January are the coldest months and intensely hot and dry weather is experienced from April through June. The study was conducted as per the guidelines laid down in the Helsinki Declaration, was approved by the institutional ethics committee of both hospitals and was registered in the clinical trials registry of the Indian Council for Medical Research (ctri.nic.in; CTRI/2012/09/002958). Parents provided written informed consent. All mothers giving birth in two maternity units of the institution, who intended to continue exclusive breast-feeding until the first 6 months and come to the hospital of birth for immunisation, were invited to participate. Exclusion criteria included (a) birth weight ≤2 kg, (b) sick neonate admitted to the intensive care unit, (c) mother or infant on treatment with anticonvulsants or antitubercular drugs and (d) mothers who had received any vitamin D other than the 10µg present in Ca tablets. At the time period of this study, there exist no recommendations for vitamin D supplementation during pregnancy or lactation by the Indian government maternal health programme.
Intervention
After maternal blood sample was collected for serum 25(OH)D 2–4 d after delivery, mother–infant pairs were randomly assigned at birth to one of three treatment regimens described below, to be followed for 9 months. Numbers were computer-generated and allocation was done by one research staff who supervised medication distribution. This staff member was not involved in data collection.
Group A (indirect supplementation)
Under supervision, mothers received oral vitamin D3 3000µg (two sachets of 1500µg each) within 7 d of delivery, and then 3000µg at 1·5, 2·5 and 3·5 months on routine immunisation visits also under supervision. At 3·5 and 6 months, mothers were provided oral vitamin D3 for the next 3 months and were instructed to take 3000µg monthly. The baby was given placebo syrup.
Group B (direct supplementation)
The baby received 10µg vitamin D preparation orally daily until 9 months. The mother was given placebo sachets.
Group C (control)
Neither the baby nor the mother was given any vitamin D supplementation. Both were given their respective placebo.
Mothers were advised to get sun exposure for at least half an hour daily. All mothers were given 500 mg of elemental Ca daily in the form of calcium carbonate, in two divided doses, in addition to instruction about dietary sources of Ca. They were instructed to give their infants 15 min of traditional baby massage once a day, under the sun between 09.00 and 16.00 hours A printed pictorial leaflet was provided to all mothers explaining the benefits of sun exposure.
Data collection
Clinic visits were planned for baby age 1·5, 2·5, 3·5, 6 and 9 months. If visits were missed, then phone calls were made to remind the mother regarding the visit. After this reminder, a research staff visited the home and administered/provided medication. At every clinic or home visit, the infant’s anthropometry, health status and adherence to medicines were documented by tablet counting and visual inspection of the syrup bottles, and the importance of sun exposure was reiterated. With regard to vitamin D or placebo sachet after the 3·5-month visit, phone calls were made on the due date, reminding the mother to take her dose. Length was measured by an infantometer (NeoCare), weight by a digital weighing machine (Salter) and head circumference by a non-stretchable nylon tape.
Demographic information including education and income were reported by the parents. Socio-economic scoring was done as per the Kuppuswamy scale( Reference Kuppuswamy 10 ), with income score updated as of September 2012( 11 ). Parents and researchers were blinded to treatment. Adherence evaluation included recording the number of missed doses of vitamin D drops and Ca tablets at each visit. Exposure to sunlight was stressed at every visit and by monthly phone calls. The type of feeding and the number of days the baby suffered respiratory or diarrhoeal illness was documented during monthly phone calls and at each visit. Diarrhoeal illness was reported telephonically by the mother and was defined as frequent stools with abnormal consistency, lasting 2 d or more. Respiratory illness was defined as the presence of rhinorrhoea, blocked nose, cough, sneeze or tachypnoea, with or without fever.
Outcome measures
The primary outcome was the infant’s serum 25(OH)D at 3·5 months. Secondary outcomes were (a) serum Ca, P, alkaline phosphatase and plasma PTH, in infants at 3·5 months of age; (b) anthropometry including weight, length, head circumference and maximum diameter of the anterior fontanelle measured at 3·5, 6 and 9 months; (c) number of days the baby suffered diarrhoeal or respiratory morbidity within 9 months of life; (d) mother’s serum 25(OH)D and calciuria at 3·5 months; and (e) number of teeth at 9 months across the three groups. All analyses were done as per protocol.
Assays
Plasma and serum samples from the mother and child were stored frozen at −80°C for analysis of PTH and 25(OH)D by immunoradiometric assay and RIA, respectively (DiaSorin). Inter-assay and intra-assay CV for PTH are 9·7 and 12·2 %, respectively, and for 25(OH)D they are 8·2–11·0 % and 8·6–12·5 %, respectively. Serum total Ca, P, alkaline phosphatase, albumin (both mother and infant) and urinary Ca and creatinine (mother) were measured (Randox) within 24 h of collection.
Sample size
The sample size of forty-nine in each group was calculated to detect a difference (effect size of 50 %) in mean 25(OH)D between groups, which gave the study 80 % power at the 0·05 significance level. Assuming 33 % dropout, seventy-five participants per group or 230 mother–infant pairs in all were recruited.
Statistical analysis
Differences among groups for continuous variables were re-tested using ANOVA for normally distributed data or Kruskal–Wallis test for non-parametric data. Post hoc comparisons were done using the Bonferroni test. Group differences for categorical variables were analysed using χ 2 or with Fisher’s exact test. Pearson’s test for normally distributed data and Spearman’s test for non-parametric data were performed for correlations. Statistical significance was set at P≤0·05 with two-tailed testing. Data were analysed using SPSS version 21.0 statistical software for Windows.
Results
Of 2563 women who delivered during the 9-month period between September 2012 and June 2013 in two units of the department, 1819 did not meet inclusion and exclusion criteria (primarily as they resided far away and intended to follow up for immunisation visits closer to their home) and 514 declined consent (Fig. 1). Of 230 recruited mother–infant pairs, 152 came for the 3·5 month visit (66 % response rate). Those who came for follow-up did not differ from those who were absent, in maternal (socio-economic score, and BMI and 25(OH)D 2–4 d after delivery) and infant (birth length, weight, head circumference, chest circumference and maximum anterior fontanelle diameter) characteristics (online Supplementary Table S1). Among those who came for follow-up, maternal and infant baseline characteristics did not differ among the groups (Table 1). There was no difference in the proportion of infants receiving formula in addition to breast milk, among the three groups, at 3·5 months (31·6, 27·4 and 27·3 % of groups A, B and C babies, respectively, P=NS). At 9 months, 90 % of infants in group A, 88 % in group B and 91 % in group C were still receiving breast milk (P=NS). Missed Ca doses by mother and vitamin D doses of the baby were documented to be 42·2 (sd 32·9) and 10·1 (sd 10·3) in group A, 36·6 (sd 39·1) and 16·3 (sd 11·9) in group B and 37·3 (sd 37·8) and 15·6 (sd 13·1) in group C (P=0·59 and 0·19, respectively).

Fig. 1 CONSORT (CONsolidated Standards of Reporting Trials) diagram. Flow of participants in the study from screening to randomisation.
Table 1 Baseline characteristics of mothers and infants (Medians and interquartile ranges (IQR); mean values and standard deviations)

25(OH)D, 25 hydroxycholecalciferol.
* Group A: mothers received oral vitamin D3 3000 μg within 7 d of delivery, and then 3000 μg at 1·5, 2·5, 3·5 months, under supervision. At 3·5 and 6 months, mothers were provided oral vitamin D3 for the next 3 months and were instructed to take 3000 μg monthly. The baby was given placebo syrup.
† Group B: the baby received 10µg vitamin D preparation orally daily. The mother was given placebo sachets.
‡ Group C (control): neither the baby nor the mother was given any vitamin D supplementation. Both were given their respective placebo.
§ BMI: both measurements 2–4 d after delivery.
║ To convert 25(OH)D from nmol/l to ng/ml, divide by 2·5.
The median 25(OH)D of infants at 3·5 months was significantly higher in groups A and B than in group C (Table 2). A similar result was obtained on analysing only those babies (n 109) who were not on fortified formula by 3·5 months. The fact that this difference is biologically relevant is suggested by the documentation of significantly higher median phosphate and lower proportion of babies with elevated alkaline phosphatase in group B than in group C infants, and significantly lower PTH in groups A and B infants than in group C infants. Infant total Ca levels did not differ among the groups, neither did the proportion of infants with hypocalcaemia. There was a moderately significant negative correlation of infant Ca with infant PTH (Spearman’s ρ −0·215, P<0·05) and a positive correlation with mother 25(OH)D (Spearman’s ρ 0·337, P<0·01).
Table 2 Biochemical parameters at 3·5 months’ follow-upFootnote * (Medians and interquartile ranges (IQR); mean values and standard deviations; numbers and percentages)

25(OH)D, 25 hydroxycholecalciferol; PTH, parathyroid hormone; ALP, alkaline phosphatase, S, serum.
a P<0·01 v. group A, b P<0·05 v. group C, c P<0·01 v. group C.
* To convert 25(OH)D from nmol/l to ng/ml, divide by 2·5; S. Ca from mmol/l to mg/dl, multiply by 4; S. P from mmol/l to mg/dl, multiply by 3·09; PTH from pmol/l to pg/ml, multiply by 9·4.
† Group A: mothers received oral vitamin D3 3000µg within 7 d of delivery, and then 3000µg at 1·5, 2·5, 3·5 months, under supervision. At 3·5 and 6 months, mothers were provided oral vitamin D3 for the next 3 months and were instructed to take 3000µg monthly. The baby was given placebo syrup.
‡ Group B: the baby received 10µg vitamin D preparation orally daily. The mother was given placebo sachets.
§ Group C (control): neither the baby nor the mother was given any vitamin D supplementation. Both were given their respective placebo.
║ n 109; thirty-five each in groups A and B and thirty-nine in group C.
At 3·5 months, infants’ 25(OH)D negatively correlated with their alkaline phosphatase (Pearson’s r −0·32, P<0·01), PTH (Spearman’s ρ −0·46, P<0·01) and mothers’ PTH (Spearman’s ρ −0·37, P<0·01) and correlated positively with mothers’ 25(OH)D (Spearman’s ρ 0·444, P<0·01). Mothers’ 25(OH)D levels correlated negatively with the infant PTH (Spearman’s ρ −0·337, P<0·01) and mother’s PTH (Spearman’s ρ −0·482, P<0·01).
Growth patterns of the infants were similar between the groups throughout the study period (Table 3). Dental growth, as evidenced by the number of teeth, was in an age-appropriate way over time, with no differences by group. The morbidity over 9 months in terms of median days in diarrhoeal and respiratory illnesses was significantly higher in group C (median 46·5; interquartile range (IQR) 14·8–73·3 d) than in group A (median 18·5; IQR 8·8–31·0 d, P<0·01) or group B (median 13·0; 7–28·5 d, P<0·05). When differences between groups were looked for separately for diarrhoeal or respiratory illness, there was no statistically significant difference. Admission to hospital was needed by thirty-seven infants: fifteen in group A and eleven each in groups B and C. The highest infant 25(OH)D was 134·8 nmol/l (53·9 ng/ml) and maternal 25(OH)D was 127·8 nmol/l (51·1 ng/ml), with only one infant and two mothers (all from group A) having levels above 125 nmol/l (50 ng/ml), which as per Institute of Medicine (IOM) may lead to potential adverse effects( Reference Ross, Manson and Abrams 9 ). Several infants in all three groups showed transient hypercalcaemia. Maternal urinary Ca:creatinine ratios remained in the normal range for all groups (group A median 0·047 (IQR 0·024–0·142) mg/mg, group B 0·043 (IQR 0·028–0·102) mg/mg and group C 0·056 (IQR 0·042–0·083 mg/mg, P=NS). Hypercalciuria (urinary Ca:creatinine ratio >0·2 mg/mg) was seen in one mother each in groups A and B. Hypercalcaemia (serum Ca >10·5 mg/dl; 2·62 mmol/l) was not seen in any mother. There were no serious adverse events related to vitamin D supplementation.
Table 3 Infants’ anthropometry at 3·5 months (Medians and interquartile ranges (IQR))
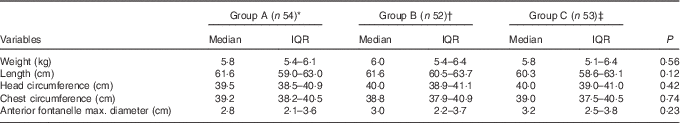
* Group A: mothers received oral vitamin D3 3000 μg within 7 d of delivery, and then 3000 μg at 1·5, 2·5, 3·5 months, under supervision. At 3·5 and 6 months, mothers were provided oral vitamin D3 for the next 3 months and were instructed to take 3000 μg monthly. The baby was given placebo syrup.
† Group B: the baby received 10 μg vitamin D preparation orally daily. The mother was given placebo sachets.
‡ Group C (control): neither the baby nor the mother was given any vitamin D supplementation. Both were given their respective placebo.
Discussion
Our findings show that one oral dose of 3000µg/month given to lactating women every month after delivery can provide adequate coverage for the breast-feeding infant up to 3·5 months. In addition, maternal supplementation greatly improved maternal vitamin D levels, thus providing for simultaneous improvement in maternal health. Provision of vitamin D directly or via breast milk was found to be superior to sunshine alone, as evidenced by improved biologically relevant parameters such as infant P and alkaline phosphatase, as well as the number of days of respiratory or diarrhoeal infection in infancy. We did not have a second control group of mothers who were not given specific sun exposure advice. Our babies in group C had higher serum 25(OH)D (mean 43·75 (sd 26) nmol/l (17·5 (sd 10·4) ng/ml) and median 45·25 nmol/l (18·1 ng/ml)) than that reported by us in cord blood previously (mean serum 25(OH)D 21 (sd 14·25) nmol/l (8·4 (sd 5·7) ng/ml)( Reference Sachan, Gupta and Das 5 ) or median serum 25(OH)D 18·5 nmol/l (7·4 ng/ml)( Reference Kalra, Das and Agarwal 12 )) or in infants aged 2–24 weeks from two other studies from Delhi (which is at a similar latitude as Lucknow) with a median serum 25(OH)D of 25·25 (IQR 6·25–42·75) nmol/l (10·1 (IQR 2·5–17·1) ng/ml)( Reference Jain, Gupta and Kalaivani 2 ) and a mean 25(OH)D of 29 (sd 20·75) nmol/l (11·6 (sd 8·3) ng/ml)( Reference Seth, Marwaha and Singla 13 ). In a recent study across three international sites, serum 25(OH)D in infants was associated with their sun index (sun exposure×body surface area exposed while outdoors)( Reference Dawodu, Davidson and Woo 14 ). However, without having a concurrent second control group that did not get repeated sun exposure advice, it is not possible to comment on whether this advice made a difference in our study. Although about 30 % of babies in all three groups were already receiving some formula feed, the vitamin D fortification of which could have contributed to their serum 25(OH)D, the trend of rising serum 25(OH)D in infancy has also been reported in studies in which babies had not received formula( Reference Challa, Ntourntoufi and Cholevas 15 ).
The next important issue is the dose of vitamin D administered to mothers and babies. Basile et al.( Reference Basile, Taylor and Wagner 16 ) studied breast-feeding mothers randomised at 1 month postpartum to vitamin D supplementation of 50 (n 12) or 100 (n 13) μg/d given for 3 months. Results showed that the 100 μg/d regimen was more efficient in raising both maternal and infant serum 25(OH)D levels and breast milk antirachitic activity than the 50µg/d regimen. It is worthy of note, however, that maternal mean serum 25(OH)D after 3 months for the 100µg/d regimen was 107·5 nmol/l (43·0 ng/ml), higher than the corresponding group receiving 3000µg/month (amounting to 100µg/d) in our study with a mean 25(OH)D of 61·5 nmol/l (24·6 ng/ml). The corresponding infant mean serum 25(OH)D at 3 months was 77 nmol/l (30·8 ng/ml), higher than that of our infants (53·75 nmol/l (21·5 ng/ml)). This difference could be because of the higher baseline maternal mean 25(OH)D of 71·25 nmol/l (28·5 ng/ml) in that group in the study by Basile et al. studies by Hollis et al.( Reference Hollis, Wagner and Howard 6 ) also confirmed a maternal dose of 6400 units/d to be adequate and safe, providing similar infant 25(OH)D as those infants given direct 10µg supplementation daily. At the time of writing, single-dose regimens have cost and potential compliance advantages in India.
A few Indian and international studies have evaluated direct infant vitamin D supplementation. Mutlu et al.( Reference Mutlu, Kusdal and Ozsu 17 ) documented that Turkish infants receiving 10µg/d vitamin D achieved a mean 25(OH)D of 106·3 (sd 64·5) nmol/l (42·5 (sd 25·8) ng/ml). No data on baseline maternal or infant 25(OH)D were available in this study. In our study, infants receiving 10µg/d achieved a much lower mean 25(OH)D of 56·5 (sd 23·5) nmol/l (22·6 (sd 9·4) ng/ml). The difference could again be because of different baseline 25(OH)D levels. In a recent study from India in preterm infants, 20µg/d of vitamin D was shown to be superior to 10µg/d at 3 months in preventing vitamin D levels <50 nmol/l (20 ng/ml) (12·5 v. 35 %); however, one infant in the 20µg group had serum 25(OH)D in the range defined as vitamin D excess (250–375 nmol/l or 100–150 ng/ml)( Reference Natarajan, Sankar and Agarwal 18 ).
The infants did not differ in anthropometric measures at any time point, across the three groups. This finding has been inconsistent in various studies of vitamin D supplementation in infancy( Reference Kumar, Sachdev and Chellani 8 , Reference Vieth Streym, Kristine Moller and Rejnmark 19 ). Over 9 months, parameters for dentition including onset of teeth and number of teeth did not differ among the groups. In a previous study, preterm infants randomised to receive a vitamin D dose of 12·5 or 25 μg/d showed that vitamin D did not affect maturation of the primary dentition in preterm children( Reference Backstrom, Aine and Maki 20 ). The role of vitamin D in defence against infections is still controversial. Some studies support such a finding( Reference Aluisio, Maroof and Chandramohan 21 ), whereas others do not( Reference Kumar, Sachdev and Chellani 8 , Reference Bergman, Lindh and Bjorkhem-Bergman 22 ).
There were no serious adverse events in any group in our study. A similar proportion of infants developed transient hypercalcaemia in all three groups. This phenomenon has been recently documented in newborns by other investigators reporting on supplementation of vitamin D in pregnancy. The hypercalcaemia was asymptomatic, and it spontaneously resolved; there is no clear explanation for it( Reference Harrington, Perumal and Al Mahmud 23 ). Notwithstanding this, the fact that one infant’s and two mothers’ serum 25(OH)D were documented to be slightly >125 nmol/l warrants that larger studies should further document safety of the maternal supplementation dose used in our study.
The strengths of our study include the randomised, placebo-controlled design and the direct supervision of vitamin D administration to mothers. The fact that the baby is unclothed during the massage allowed us to make a serious attempt at harnessing the abundant sunshine. The results of parameters such as PTH, P and alkaline phosphatase highlight the biological relevance of the vitamin supplementation. An important limitation of our study is the 34 % dropout at primary outcome, with a potential for bias in the results. Although a significant proportion of infants received formula feeds in addition to breast milk, this was similar in the three groups and may not have influenced the outcome. We were unable to quantify the exact quantities of formula being consumed by each infant, but babies not receiving any formula at 3·5 months had almost identical 25(OH)D as those receiving it. Third, the duration of infections did not remain significantly different when respiratory and diarrhoeal infections were separately analysed; a larger study is needed before a firm conclusion can be drawn regarding this outcome. Last, no X rays were performed on the infants who had elevated alkaline phosphatase, to look for rickets.
In summary, our findings suggest that an oral dose of 3000µg/month cholecalciferol given to lactating women is equivalent to daily dosing of the infant with 10µg in raising infant serum 25(OH)D at 3·5 and improving relevant biological indices. Both dose regimens, given along with sun exposure, are superior to traditional durations of sun exposure alone in maintaining normal infant 25(OH)D at 3·5 months, and providing protection from elevated alkaline phosphatase and possibly from infectious morbidity. These doses of vitamin D correspond to the IOM-recommended maximum daily upper limit of intake of 100µg for lactating women, but the single monthly dosing lends itself to administration under observation and benefits both mother and child. These findings, including the safety of the maternal dose, need confirmation in other settings and populations.
Acknowledgements
The authors thank Anshu Srivastava, Arjun Singh, Pallavi Tiwari and Abhishek Kumar for their help in conduct of the study.
This work was supported by grants to V. B. from the Department of Biotechnology, Government of India (BT/PR13985/SPD/11/297/2010) and the Indian Society for Bone and Mineral Research. The funding agencies had no role in the study design; the collection, analysis and interpretation of data; the writing of the report; and the decision to submit the paper for publication.
D. D. C. planned and conducted the study, analysed the data and wrote the first draft of the manuscript; J. K. enrolled study subjects, conducted the study, analysed data and wrote the first draft of the manuscript; S. N. S. conceptualised the study, supervised all details, analysed the data and edited the manuscript; A. A. and U. S. supervised subject enrolment and acquisition of data and approved the manuscript; V. D. planned the conduct of the study and edited the manuscript; V. R. supervised conduct of the study and approved the manuscript; V. B. conceptualised the study, planned and supervised its conduct, analysed the data and edited the manuscript and will take guarantee for the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516001756







