The innate immune system is the first line of defence against invading pathogens. Innate immune responses are initiated by pattern recognition receptors, which recognise specific structures expressed by micro-organisms( Reference Kawai and Akira 1 – Reference Takeuchi and Akira 3 ). Retinoic acid-inducible gene I (RIG-I), a type of pattern recognition receptor, plays a vital role in cytoplasmic viral double-stranded RNA recognition and innate immunity and in bridging innate and adaptive immune responses( Reference Yoneyama, Kikuchi and Natsukawa 4 , Reference Yoneyama, Kikuchi and Matsumoto 5 ). Upon recognition, RIG-I triggers signals through interferon-β promoter stimulator 1 (IPS-1, also termed MAVS/VISA/Cardif), which results in the activation of host transcription factors, including NF-κB and interferon regulatory factor 3, that orchestrate an early interferon (IFN)-independent transcriptional programme( Reference Kawai, Takahashi and Sato 6 – Reference Xu, Wang and Han 8 ). Innate immune responses to viral infections are critically dependent on the successful production of type I IFN( Reference Randall and Goodbourn 9 ).
Vitamin D belongs to the steroid hormone family. Vitamin D has two major forms, 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D3 (1,25(OH)2D3). The major storage form of vitamin D is 25-hydroxyvitamin D, which is converted by the enzyme 1α-hydroxylase (CYP27B1, cytochrome P450, family 27, subfamily B, polypeptide 1) to the main active metabolite 1,25(OH)2D3( Reference Prosser and Jones 10 ). Vitamin D has conventionally been associated with bone mineralisation and Ca homeostasis. New evidence from epidemiological studies reveals that it also plays an important role in the regulation of the immune system, including immune responses to viral infections. Vitamin D deficiency may predispose individuals to an increased risk of viral infections such as influenza( Reference Urashima, Segawa and Okazaki 11 ), respiratory tract infections( Reference Hansdottir, Monick and Lovan 12 ) and HIV( Reference Teichmann, Stephan and Lange 13 ). A recent study has demonstrated that vitamin D has a direct anti-hepatitis C virus effect in an ‘in vitro’ infectious virus production system( Reference Gal-Tanamy, Bachmetov and Ravid 14 ). These results indicate that vitamin D mediates innate antiviral immune responses.
Rotavirus, a double-stranded RNA icosahedral RNA virus, is a major pathogen causing severe gastroenteritis in infants and young children, as well as in other young animals( Reference Donelli and Superti 15 ). Recent studies have shown that the activation of the antiviral response by rotavirus depends on the RIG-I signalling pathway in vitro ( Reference Broquet, Hirata and McAllister 16 , Reference Sen, Pruijssers and Dermody 17 ). However, there are limited studies that have been carried out in vivo. As vitamin D represents a potentially useful intervention agent for combating viral infections, we assumed that the vitamin D-mediated innate antiviral immune response might be associated with the regulation of the RIG-I signalling pathway. Thus, we conducted the present study by employing a pig model of rotavirus infection to test this hypothesis.
Materials and methods
Animals and housing
The present experiment was carried out at the Experimental Farm of Sichuan Agricultural University. The animals were cared for according to the laws and regulations governing experiments with live animals in China. A total of twenty-four Duroc × Landrance × Yorkshire cross-bred pigs with an initial body weight of 21·82 (sem 2·06) kg were randomly allocated to four treatment groups. The pigs were placed individually in metabolism cages (1·5 × 0·7 × 1·0 m) with a self-feeder and a nipple watering device, which were located in a temperature (25 ± 1°C)-controlled room. All the diets (Table 1) were formulated according to the National Research Council (NRC, 1998) requirements for all nutrients( 18 ).
Table 1 Composition of the diets used in the present study (as-fed basis)

* The two experimental diets were supplemented with 200 IU of vitamin D3 (control) or 5000 IU of vitamin D3; where 1 IU of vitamin D is defined as the biological activity of 0.025 mg of cholecalciferol.
† Vitamin premix provided the following (per kg of diet): vitamin A, 5512 IU; vitamin D3, 200 or 5000 IU; vitamin E, 24 mg; vitamin K3, 3 mg; vitamin B1, 3 mg; vitamin B2, 6 mg; vitamin B6, 3 mg; vitamin B12, 24 μg; d-pantothenic acid, 15 mg; nicotinic acid, 30·3 mg; folic acid, 1·2 mg; biotin, 150 μg.
‡ Mineral premix provided the following (per kg of diet): Fe, 60 mg; Cu, 4 mg; Zn, 60 mg; Mn, 2 mg; Se, 0·10 mg; I, 0·14 mg.
§ Nutrient levels represent the calculated values.
The pigs were fed the experimental diets for 7 d before the porcine rotavirus (PRV) challenge. Before the pigs were challenged with PRV, the experiment had a randomised complete-block design. After the pigs were challenged, the experiment was conceived as a 2 × 2 factorial arrangement based on the factors of challenge status (PRV challenged and unchallenged) and dietary vitamin D concentrations (200 and 5000 IU; where 1 IU of vitamin D is defined as the biological activity of 0.025 mg of cholecalciferol). In addition, the pigs challenged with PRV were fed separately from those orally dosed with essential medium to prevent mutual infection. Random distribution of two experimental diets was done between the pens of each room.
Rotavirus preparation
PRV (purchased from China Institute of Veterinary Drug Control) was a tissue culture-adapted Ohio State University (OSU) strain (ATCC #VR-893). The virus was propagated in IPEC-J2 cells as described previously( Reference Liu, Li and Wen 19 ). The virus was pre-activated with 5 μg/ml trypsin (type IX, Sigma Chemical Company) for 30 min at 37°C. The IPEC-J2 cells were inoculated with pre-activated rotavirus. After 60 min of incubation at 37°C, the inoculums were removed, and the monolayers were washed three times with PBS and then incubated at 37°C in Dulbecco's modified Eagle's medium/Ham's F-12 medium (DMEM/F12 medium). When an extensive cytopathic effect was observed by microscopy, the infected cultures were frozen and thawed three times and centrifuged (3000 g for 10 min), and supernatants containing the virus were stored at − 80°C.
Virus titre determination
The tissue culture infective dose 50 (TCID50) value was determined exactly as described previously( Reference Botic, Klingberg and Weingartl 20 ). Briefly, IPEC-J2 cells grown to 80–100 % confluency in ninety-six-well plates were infected with 50 μl aliquots of 1:10 serial dilutions (in DMEM/F12 medium) of virus samples (eight wells per dilution). After incubation for 4 d at 37°C in 5 % CO2, cytopathic effects (cell destruction) were visualised by staining the remaining viable cells with crystal violet. Virus titres were calculated according to the method of Spearman( Reference Spearman 21 ) and are expressed as log10(TCID50).
Rotavirus infection model
All animal procedures were approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University. The pigs were fed the experimental diets in the first week of the study period. To prevent intragastric viral inactivation, all the pigs were administered 5 ml of a 100 mm solution of sterile sodium bicarbonate before the PRV challenge( Reference Graham, Dufour and Estes 22 ). On day 8 of the dietary treatments, the challenged pigs were orally dosed with 4 ml (105·8 TCID50/100 μl) of rotavirus OSU strain and the unchallenged pigs with 4 ml of essential medium. The pigs were checked daily to evaluate their status after rotavirus challenge. Clinical signs (i.e. dehydration, apathy and diarrhoea) were monitored daily. Faecal consistency was recorded each morning at 08.00 hours by visual appraisal of each sample using a four-point scoring system and scored as follows: 0, solid; 1, semi-solid; 2, semi-liquid; 3, liquid. Infection was defined as the excretion of rotavirus in the faeces with or without the development of clinical symptoms, usually diarrhoea.
Blood samples were collected in the morning of days 8 and 14 and were centrifuged at 3000 g for 10 min. The isolated serum samples were stored at − 20°C until analysis. On day 14, all the pigs were killed with an intracardiac injection of sodium pentobarbital (150 mg/kg). The abdomen was immediately opened. The intestinal sections were identified according to the method of Wang et al. ( Reference Wang, Gu and Tang 23 ). The mid-jejunum was isolated for histological analysis. The duodenum, jejunum and ileum were removed, flushed with ice-cold saline and then snap-frozen in liquid N2 for further analysis.
Cytokine assays
The serum concentrations of IL-2, IL-6 and IFN-β were determined using commercially available swine ELISA kits (R&D Systems China Company Limited) according to the manufacturer's instructions. The detection limits of these ELISA were as follows: IL-2, 8 pg/ml; IL-6, 8 pg/ml; IFN-β, 20 pg/ml. The serum concentration of 1,25(OH)2D3 was determined by ELISA (Immunodiagnostic Systems, Inc.). All the assays were run in duplicate using a plate reader.
Total RNA extraction and reverse transcription reaction
Total RNA was isolated using the TRIzol reagent (Invitrogen) according to the manufacturer's instructions. The concentrations of total RNA were determined spectrophotometrically using a Beckman Coulter DU 800 (Beckman Coulter). The integrity of the RNA was verified by agarose gel electrophoresis. From each sample, 1 μg of total RNA was reverse-transcribed in a final volume of 20 μl using the PrimeScript® RT Reagent Kit with gDNA Eraser (TaKaRa) according to the manufacturer's protocol. RT products (complementary DNA) were stored at − 70°C.
Real-time quantitative PCR
Real-time quantitative PCR were carried out using the CFX96 Real-Time PCR Detection System (Bio-Rad). The gene-specific primers used in the present study are listed in Table 2. The PCR mixture consisted of l μl of the first-strand complementary DNA sample, 0·5 μl of each of the forward and reverse primers from 10 μm stocks, 3 μl of diethylpyrocarbonate-treated water and 5 μl of 2 × SsoFast EvaGreen Supermix (Bio-Rad).Cycling conditions were as follows: 98°C for 10 s, followed by forty cycles of 98°C for 5 s, annealing at temperatures given in Table 2 for 10 s and 72°C for 15 s. A melting curve analysis was carried out after each real-time quantitative PCR to check and verify the specificity and purity of all PCR products. Each sample was amplified in triplicate. A standard curve was constructed using serial dilutions of one of the complementary DNA samples. The standard curve was drawn by plotting the natural log of the threshold cycle (C t) against the natural log of the number of molecules. The C t was defined as the cycle at which a statistically significant increase in the magnitude of the signal generated by the PCR was first detected. The standard curve of each gene was generated in duplicate and three times for obtaining reliable amplification efficiency values. The correlation coefficients (r 2) of all the standard curves were >0·99 and the amplification efficiency values ranged between 90 and 110 %. The mRNA concentration of the target gene was normalised to that of the reference gene β-actin.
Table 2 Primers and annealing temperatures used in real-time quantitative PCR

RIG-I, retinoic acid-inducible gene I; QF, quantitative forward primer; QR, quantitative reverse primer; TLR3, Toll-like receptor 3; IPS-1, interferon-β promoter stimulator 1; ISG15, interferon-stimulated gene 15; IFN, interferon; PRV, porcine rotavirus; CYP27B1, cytochrome P450, family 27, subfamily B, polypeptide 1; ACT, actin.
Statistical analysis
Data collected before the PRV challenge were analysed by one-way ANOVA using the general linear model procedure of the Statistical Analysis Package, SAS version 9.1 (SAS Institute, Inc.). Faecal consistency data were analysed as repeated measures using the MIXED procedure of SAS 9.1 according to the following model:
where μ is the mean, α i is the effect of immune challenge (i= PRV or essential medium), β j is the effect of vitamin D (j= 200 or 5000 IU), αβ ij is the interaction between immune challenge and vitamin D, U k is the pig (k= 1, 2,…, 24), ω l is the time (days 1, 2,…, 6), αω il is the interaction between immune challenge and time, βω jl is the interaction between vitamin D and time, αβω ijl is the interaction between immune challenge, vitamin D and time, and ɛ ijkl represents the random error. Growth performance, intestinal morphology, serum cytokine and gene expression data were analysed according to the model, but the effects of time and the interaction between time, immune challenge and vitamin D were omitted. When the effects of treatment were established (P< 0·05), treatment least-squares means were separated using the probability of difference function adjusted by the Tukey–Kramer test. Random effect was used to account for the variation between the pigs. Results are expressed as least-squares means with their standard errors. Probability values < 0·05 were used as the criterion for statistical significance.
Results
Growth performance
During the pre-challenge periods, there were no significant differences in the average daily gain, average daily feed intake (ADFI) and feed:gain ratio of the pigs. Compared with the unchallenged pigs, the 200 IU dietary vitamin D-supplemented pigs challenged with PRV had decreased average daily gain (P <0·05) and ADFI (P <0·05), whereas in the 5000 IU dietary vitamin D-supplemented pigs challenged with PRV, average daily gain and ADFI reduction was significantly alleviated (Table 3). The PRV challenge also led to a remarkable decrease in the feed:gain ratio (P <0·01). However, there were no significant effects of dietary supplementation with 5000 IU vitamin D on the feed:gain ratio. There was an interaction between dietary vitamin D levels and PRV challenge with regard to ADG and ADFI.
Table 3 Effect of vitamin D (VD) supplementation and porcine rotavirus (PRV) challenge on the growth performance of pigs* (Mean values with their standard errors, 1–7 d (n 12); 8–14 d (n 6))
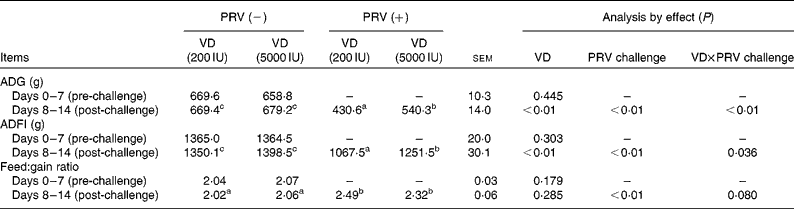
ADG, average daily gain; ADFI, average daily feed intake.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* 1 IU of vitamin D is defined as the biological activity of 0.025 mg of cholecalciferol.
Faecal consistency
The effects of treatment on faecal consistency from day 8 to day 14 after the challenge are shown in Fig. 1. On day 6 after the challenge, the 200 IU dietary vitamin D-supplemented pigs challenged with PRV had reduced faecal consistency compared with the unchallenged pigs (P <0·05). Dietary supplementation with 5000 IU vitamin D improved the faecal consistency of the challenged pigs compared with dietary supplementation with 200 IU vitamin D, but not to the same extent as that observed in the unchallenged pigs (P <0·05).
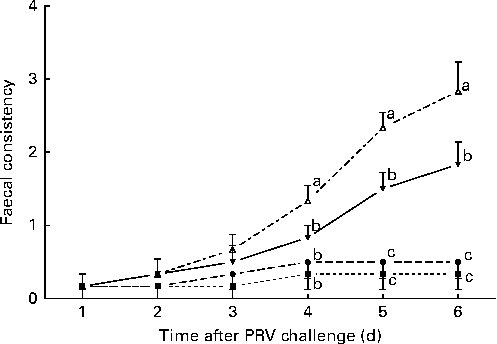
Fig. 1 Faecal consistency (experimental days 1–6) of pigs not challenged with PRV and fed a diet supplemented with 200 IU vitamin D (NONV, ![]() ), pigs not challenged with PRV and fed a diet supplemented with 5000 IU vitamin D (NOHV,
), pigs not challenged with PRV and fed a diet supplemented with 5000 IU vitamin D (NOHV, ![]() ), pigs orally dosed with porcine rotavirus (PRV) and fed a diet supplemented with 200 IU vitamin D (CHNV,
), pigs orally dosed with porcine rotavirus (PRV) and fed a diet supplemented with 200 IU vitamin D (CHNV, ![]() ), and pigs orally dosed with PRV and fed a diet supplemented with 5000 IU vitamin D (CHHV,
), and pigs orally dosed with PRV and fed a diet supplemented with 5000 IU vitamin D (CHHV, ![]() ); where 1 IU of vitamin D is defined as the biological activity of 0.025 mg of cholecalciferol. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different within each day (P< 0·05).
); where 1 IU of vitamin D is defined as the biological activity of 0.025 mg of cholecalciferol. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different within each day (P< 0·05).
Histomorphology
Compared with the unchallenged pigs, the 200 IU dietary vitamin D-supplemented pigs challenged with PRV had decreased villus height (P <0·05) and crypt depth (P <0·05) (Table 4). Dietary supplementation with 5000 IU vitamin D mitigated the challenge-induced damage to the intestinal epithelium (P <0·05). There was an interaction between dietary vitamin D levels and PRV challenge with regard to villus height (P <0·01). No difference was found in the crypt depth and villi:crypt ratio in the mid-jejunum among the treatment groups.
Table 4 Effect of vitamin D (VD) supplementation and porcine rotavirus (PRV) challenge on the villus height (VH; μm), crypt depth (CD; μm) and villi:crypt ratio (VCR) in the mid-jejunum of pigs* (Mean values with their standard errors, n 6)

a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* 1 IU of vitamin D is defined as the biological activity of 0.025 mg of cholecalciferol.
Serum IL-2, IL-6 and interferon-β concentrations
As shown in Fig. 2, the 200 IU dietary vitamin D-supplemented pigs challenged with PRV had significantly increased serum concentrations of IL-2 (P <0·01), IL-6 (P <0·01) and IFN-β (P <0·01) on day 14 compared with the unchallenged pigs. Dietary supplementation with 5000 IU vitamin D decreased the concentrations of IL-2 (P <0·01) and IL-6 (P <0·01), but significantly increased that of IFN-β (P <0·01) compared with dietary supplementation with 200 IU vitamin D.
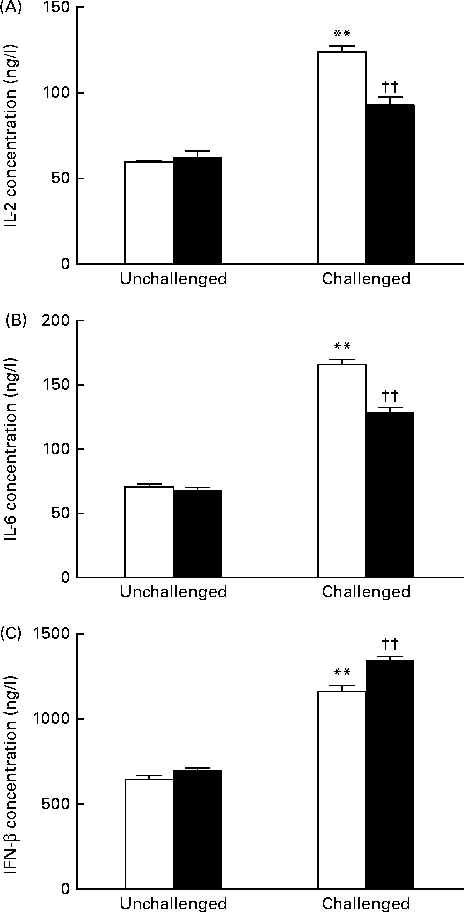
Fig. 2 Effect of vitamin D on the serum concentrations of (A) IL-2, (B) IL-6 and (C) interferon-β (IFN-β) of pigs challenged or not challenged with porcine rotavirus. □, 200 IU vitamin D; ■, 5000 IU vitamin D; where 1 IU of vitamin D is defined as the biological activity of 0.025 mg of cholecalciferol. Values are means, with their standard errors represented by vertical bars. ** Mean value was significantly different from that of the 200 IU vitamin D-supplemented group not challenged with PRV (P< 0·01). †† Mean value was significantly different from that of the 200 IU vitamin D-supplemented group challenged with PRV (P< 0·01).
Serum 1,25-dihydroxyvitamin D concentration and cytochrome P450, family 27, subfamily B, polypeptide 1 mRNA expression in the mid-jejunum
Data obtained for serum 1,25(OH)2D3 concentration and CYP27B1 mRNA expression in the mid-jejunum are shown in Fig. 3. Compared with the 200 IU dietary vitamin D-supplemented pigs not challenged with PRV, the 200 IU dietary vitamin D-supplemented pigs challenged with PRV had a reduced serum concentration of 1,25(OH)2D3 (P <0·01). Dietary supplementation with 5000 IU vitamin D increased the serum concentration of 1,25(OH)2D3 (P <0·01). CYP27B1 is required for the conversion of 25-hydroxyvitamin D into 1,25(OH)2D3. The abundance of CYP27B1 mRNA in the mid-jejunum was enhanced after the PRV challenge (P <0·01). Dietary supplementation with 5000 IU vitamin D tended to increase the abundance of CYP27B1 mRNA in the mid-jejunum. Furthermore, there was a significant effect of vitamin D level × PRV challenge interaction on the abundance of CYP27B1 mRNA in the mid-jejunum.
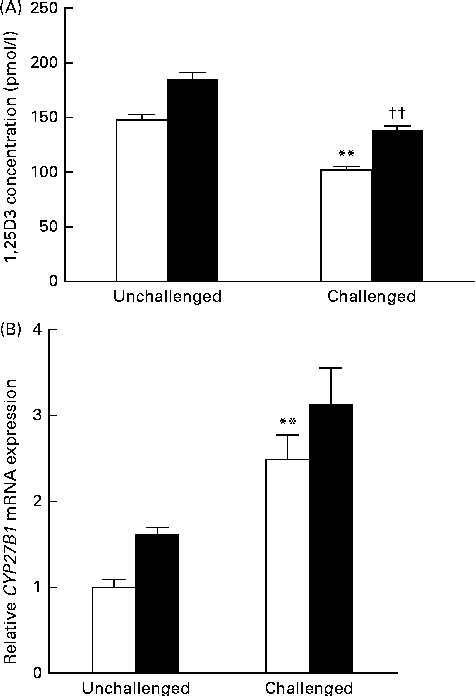
Fig. 3 Effect of vitamin D on the (A) serum 1,25-dihydroxyvitamin D (1,25(OH)2D3) concentration and (B) cytochrome P450, family 27, subfamily B, polypeptide 1 (CYP27B1) mRNA expression of pigs challenged or not challenged with porcine rotavirus. □, 200 IU vitamin D; ■, 5000 IU vitamin D; where 1 IU of vitamin D is defined as the biological activity of 0.025 mg of cholecalciferol. Values are means, with their standard errors represented by vertical bars. ** Mean value was significantly different from that of the 200 IU vitamin D-supplemented group not challenged with PRV (P< 0·01). †† Mean value was significantly different from that of the 200 IU vitamin D-supplemented group challenged with PRV (P< 0·01).
Expression of retinoic acid-inducible gene I, Toll-like receptor 3, interferon-β promoter stimulator 1, interferon-β and interferon-stimulated gene 15 in the intestine
Data obtained for the mRNA expression of RIG-I, Toll-like receptor (TLR)3, IPS-1, IFN-β and ISG 15 are summarised in Table 5. Compared with the 200 IU dietary vitamin D-supplemented pigs not challenged with PRV, the 200 IU dietary vitamin D-supplemented pigs challenged with PRV had an increased abundance of RIG-I mRNA in the duodenum (P <0·05), jejunum (P <0·05) and ileum (P <0·05). Dietary supplementation with 5000 IU vitamin D significantly increased the abundance of RIG-I mRNA in the jejunum (P <0·05) and ileum (P <0·05) compared with dietary supplementation with 200 IU vitamin D. There was a significant effect of vitamin D level × PRV challenge interaction on the mRNA expression of RIG-I in the jejunum and ileum (P< 0·01). No effects of treatment were observed on the mRNA expression of TLR3.
Table 5 Effect of vitamin D (VD) supplementation and porcine rotavirus (PRV) challenge on the relative expression of retinoic acid-inducible gene I (RIG-I), Toll-like receptor (TLR3), interferon-β promoter stimulator 1 (IPS-1), interferon-β (IFN-β) and interferon-stimulated gene 15 (ISG 15 ) mRNA in the intestine of pigs* † (Mean values with their standard errors, n 6)

a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* Tissues were collected in the morning of day 14.
† 1 IU of vitamin D is defined as the biological activity of 0.025 mg of cholecalciferol.
Compared with the 200 IU dietary vitamin D-supplemented pigs not challenged with PRV, the 200 IU dietary vitamin D-supplemented pigs challenged with PRV had an increased abundance of IPS-1 mRNA in the duodenum (P <0·05), jejunum (P <0·05) and ileum (P <0·05). Dietary supplementation with 5000 IU vitamin D significantly increased the abundance of RIG-I mRNA in the duodenum (P <0·05), jejunum (P <0·05) and ileum (P <0·05) compared with dietary supplementation with 200 IU vitamin D. There was a significant effect of vitamin D level × PRV challenge interaction on the mRNA expression of IPS-1 in the jejunum (P< 0·01).
After the PRV challenge, the mRNA expression of IFN-β was increased in the duodenum (P< 0·01), jejunum (P< 0·01) and ileum (P< 0·01). Compared with the 200 IU dietary vitamin D-supplemented pigs challenged with PRV, the 5000 IU dietary vitamin D-supplemented pigs challenged with PRV had an increased abundance of IPS-1 mRNA in the duodenum (P <0·05), jejunum (P <0·05) and ileum (P <0·05). There was a significant effect of vitamin D level × PRV challenge interaction on the mRNA expression of IFN-β in the duodenum (P <0·01) and jejunum (P <0·05).
The abundance of ISG 15 mRNA in the duodenum, jejunum, and ileum (P< 0·01) was enhanced after the PRV challenge. Compared with the 200 IU dietary vitamin D-supplemented pigs challenged with PRV, the 5000 IU dietary vitamin D-supplemented pigs challenged with PRV had an increased abundance of IPS-1 mRNA in the jejunum (P <0·05) and ileum (P <0·05). There was no effect of vitamin D level × PRV challenge interaction.
Discussion
Rotavirus is a major pathogen causing symptomatic gastroenteritis in infants and young children, as well as in other young animals( Reference Cukor and Blacklow 24 , Reference Barnett 25 ). The PRV used in the present experiment was a tissue culture-adapted OSU strain (ATCC #VR-893), which was passaged in IPEC-J2 cells. In the present study, we first used in vivo methods to elucidate the early interferon response to rotavirus infection and the effect of vitamin D on host–pathogen interactions.
The results of the present experiment were consistent with previous observations showing that PRV challenge caused a reduction in villus height, an increase in cytokine concentrations and finally a decrease in growth performance( Reference Boshuizen, Reimerink and Korteland-van 26 – Reference Osborne, Haddon and Spencer 28 ). Rotavirus replicates in the enterocytes of the small intestine, inducing villous atrophy and malabsorptive diarrhoea( Reference Boshuizen, Reimerink and Korteland-van 26 ). Cytokines such as IL-6 are important markers of the progress of rotavirus infection in children( Reference Jiang, Snipes-Magaldi and Dennehy 29 ). In the present experiment, the serum concentration of IL-6 was significantly increased after the PRV challenge, while faecal consistency was significantly decreased. This result indicates that the PRV challenge model is useful for studying vitamin D-mediated innate antiviral immune responses.
Vitamin D is a steroid hormone that has long been known for its role in mineral metabolism and bone growth. The main active vitamin D (1,25(OH)2D3) is thought to be a key component of non-classical vitamin D functions. In the present study, dietary vitamin D supplementation was found to increase serum 1,25(OH)2D3 concentration, while the PRV challenge was found to significantly decrease it, indicating that more amounts of 1,25(OH)2D3 are required to confer protection against viral infections in pigs. Apart from playing a role in bone mineralisation and Ca homeostasis, vitamin D also modulates the adaptive immune system by exerting direct effects on the activation of T cells and the phenotype and function of antigen-presenting cells, particularly of dendritic cells( Reference Kamen and Tangpricha 30 ). In addition, vitamin D also plays an important role in the regulation of the immune system, including immune responses to viral infections( Reference Urashima, Segawa and Okazaki 11 , Reference Hansdottir, Monick and Lovan 12 , Reference Teichmann, Stephan and Lange 13 ). Due to the functions of vitamin D, it is easy to understand why pigs need more amounts of vitamin D under immune challenge conditions. The enzyme CYP27B1 is the key determinant in the synthesis of active vitamin D. This enzyme was first detected in the kidneys, where it has been found to be highly expressed. Extrarenal CYP27B1 has since been detected in numerous tissues, including the epithelial cells at various barrier sites( Reference Bell 31 ) The presence of CYP27B1 in extrarenal tissues has been proposed as a mechanism by which vitamin D may exert a host of protective actions within these tissues( Reference Hansdottir, Monick and Hinde 32 ). In the present experiment, the mRNA expression of CYP27B1 in the mid-jejunum was also increased after the PRV challenge. This result is in agreement with that reported by Hansdottir et al. ( Reference Hansdottir, Monick and Hinde 32 ). Hansdottir et al. ( Reference Hansdottir, Monick and Hinde 32 ) reported that polyinosinic-polycytidylic acid-treated primary airway epithelial cells have a significantly higher expression of CYP27B1 mRNA than untreated cells. However, dietary supplementation with 5000 IU vitamin D in pigs challenged with PRV improved challenge-induced symptoms, as indicated by attenuated growth depression, decreased diarrhoea incidence, increased serum 1,25(OH)2D3 concentration and decreased intestinal damage. These results indicate that vitamin D has protective effects in pigs under PRV challenge conditions. In addition, the protective effects of vitamin D were also observed in vitro. Khoo et al. ( Reference Khoo, Chai and Koenen 33 ) reported that vitamin D down-regulates the proinflammatory cytokine response to Mycobacterium tuberculosis through pattern recognition receptors.
Mammalian innate immune responses to viral infections are critically dependent on a successful type I IFN response( Reference Randall and Goodbourn 9 , Reference Smith, Lombardi and Foster 34 , Reference Hiscott 35 ). Type I IFN mediates antiviral activity by triggering the expression of IFN-stimulated genes, the products of which have diverse antiviral activities. RIG-I signalling is the main arm of innate immunity under double-stranded RNA virus challenge conditions( Reference Broquet, Hirata and McAllister 16 , Reference Sen, Pruijssers and Dermody 17 , Reference Hirata, Broquet and Menchen 36 ). In the present experiment, the mRNA expression of RIG-I, IPS-1, IFN-β and ISG 15 in the intestine was up-regulated to various degrees after the PRV challenge. This is in close agreement with previous reports that RIG-I signalling through IPS-1 is required for the activation of IFN-β production by rotavirus-infected intestinal epithelial cells or mouse embryonic fibroblast cells and has a crucial role in the determination of the magnitude of rotavirus replication in the intestinal epithelium( Reference Broquet, Hirata and McAllister 16 , Reference Sen, Pruijssers and Dermody 17 ). To our knowledge, the present study is the first to demonstrate that the activation of the antiviral response by rotavirus is dependent on the RIG-I signalling pathway in vivo.
We also found that vitamin D is closely related to the RIG-I signalling pathway, which mediates innate immunity responses. The detection of pathogen-associated molecular patterns by pattern recognition receptors is critical for the initiation and coordination of immune responses against viral infections. To date, only the TLR have been reported to be associated with the immunological activities of vitamin D. A previous study has shown that the activation of TLR2/TLR1 on human macrophages increases the synthesis of 1,25(OH)2D3 by these cells. This consequently induces an increase in the concentration of the antimicrobial peptide cathelicidin, leading to the destruction of intracellular M. tuberculosis ( Reference Liu, Stenger and Li 37 ). Ovsyannikova et al. ( Reference Ovsyannikova, Dhiman and Haralambieva 38 ) reported the first evidence that polymorphisms in vitamin D receptor and RIG-I genes influence rubella vaccine-induced cellular immunity. In the present experiment, dietary supplementation with 5000 IU vitamin D increased the mRNA expression of RIG-I, IPS-1, IFN-β and ISG 15 . Thus, the present results also suggest that the immunomodulatory effects of 1,25(OH)2D3 on rotavirus arise from its influence on the RIG-I signalling pathway. However, how vitamin D interacts with the RIG-I signalling pathway is not known still and requires further study.
In conclusion, dietary vitamin D supplementation attenuated weight gain and feed intake reduction caused by the PRV challenge. It is possible that the protective effects of vitamin D in pigs challenged with PRV are associated with the activation of the RIG-I signalling pathway through the up-regulation of RIG-I, IPS-I, IFN-β and ISG 15 mRNA expression.
Acknowledgements
The authors thank Dr Jun Jiang for his assistance in the preparation of the figures. The present study was supported by the Program for ChangJiang Scholars and Innovative Research Team in the University, Ministry of Education of China (IRTO555), and the earmarked fund for the China Agriculture Research System (CARS-36) to D. C.
The authors' contributions are as follows: Y. Z. conducted the animal trial, performed the RT-PCR and ELISA experiments, and wrote the manuscript; B. Y. and X. M. contributed to the design of the study; J. H. and Z. H. assisted with the preparation of the manuscript; P. Z. and J. Y. assisted with all data analyses; G. H. and X. L. assisted with the animal trial.
None of the authors has any conflicts of interest to declare.










