Vitamin D is best known for its role in Ca homeostasis. Ageing affects both vitamin D metabolism and Ca homeostasis, with important consequences. In the present review, we outline new insights into the effects of ageing on both the vitamin D endocrine system and Ca homeostasis, which are relevant for clinicians who treat older people. Furthermore, considerations for vitamin D supplementation will be discussed.
Vitamin D metabolism
Vitamin D is a fat-soluble, seco-steroid hormone. The term vitamin D refers to two precursors, i.e. cholecalciferol and ergocalciferol. Cholecalciferol is mostly formed in the skin after exposure to sunlight. In the skin, the precursor 7-dehydrocholesterol is transformed into cholecalciferol under the influence of short-wave UV light(Reference Lips1). Another source of vitamin D is the diet. Ergocalciferol is generated in yeast and plants and cholecalciferol is produced in fish and mammals. In general, oral vitamin D intake, especially in Europe, is low and depends mostly on cutaneous production of vitamin D for our reserves(Reference Lips2). The inert precursors are transported to the liver, where they are converted to 25-hydroxyvitamin D3 (25OHD3). In the kidney, 25OHD3 is hydroxylated by the enzyme 25OHD3-1α-hydroxylase (1α-OHase) to form 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), the most active vitamin D metabolite (Fig. 1). 1α-OHase expression is not restricted to the kidney. Several cell types like macrophages, osteoblasts and neurons have also been shown to express 1α-OHase (Table 1) (Reference Hewison3–Reference McCann and Ames5).
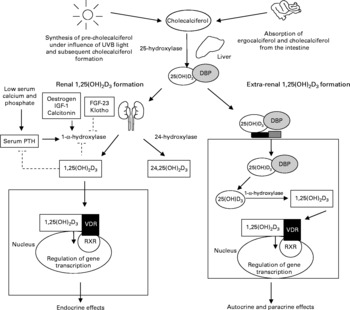
Fig. 1 Different pathways for the activation of vitamin D. Inert vitamin D precursors are either formed in the skin after exposure to UVB or derived form the diet. These precursors are hydroxylated in the liver to form 25-hydroxyvitamin D3 (25(OH)D3). The 25(OH)D3 is bound to the vitamin D-binding protein (DBP). The final 1-α-hydroxylation step that forms the most active vitamin D metabolite, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) occurs either in the kidney (bulk) or in the extra-renal cells expressing the 1-α-hydroxylase enzyme. The 1,25(OH)2D3 formation in the kidney is tightly regulated via a feedback mechanism and the active vitamin D formed in the kidney exerts endocrine effects after binding to the vitamin D receptor (VDR). The VDR forms a heterodimer with the retinoid receptor (RXR) and regulates gene transcription. The active vitamin D formed extra-renally exerts paracrine and autocrine effects. IGF, insulin-like growth factor; FGF, fibroblast growth factor; PTH, parathyroid hormone; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3.
Table 1 Extra-renal expression of the 1α-hydroxylase (1α-OHase) enzyme and effects of potential regulators relevant for ageing*

*Based on ex vivo, in vitro and animal studies.
1,25(OH)2D3, 1,25-dihydroxyvitamin D3; TGFβ1, transforming growth factor β1; IFN-γ, interferon-γ; PTH, parathyroid hormone; EGF, epidermal growth factor; ↑ , stimulating effect; ↓ , inhibiting effect; –, no effect; (?), uncertain.
The primary function of the vitamin D endocrine system is maintaining Ca and phosphate homeostasis. Vitamin D stimulates both intestinal absorption and renal reabsorption of Ca and phosphate. Vitamin D deficiency results in decreased Ca and phosphate (re)absorption and subsequently lower serum levels of Ca and phosphate. This stimulates parathyroid hormone (PTH) secretion from the parathyroid glands(Reference Holick6). PTH stimulates renal 1α-OHase expression and 1,25(OH)2D3 formation. PTH also stimulates osteoclast formation (osteoclastogenesis). Osteoclasts stimulate bone resorption, releasing Ca and phosphate ions from the bone into the blood. A recent animal study has demonstrated that osteoclastogenesis was increased in mice when serum 25OHD3 levels were < 80 nmol/l and this was positively associated with the receptor activator for NF-κB ligand/osteoprotegerin ratio. This increase in bone resorption was associated with the development of osteopenia and osteoporosis(Reference Anderson, Sawyer and Moore7). The optimal serum 25OHD3 level in human subjects to prevent stimulation of osteoclastogenesis is also believed to be about 80 nmol/l(Reference Anderson, Sawyer and Moore7).
Other hormones that are known to stimulate renal 1α-OHase expression are insulin-like growth factor 1, calcitonin and oestrogen(Reference Gomez8, Reference Gallagher, Riggs and DeLuca9). Increases in serum Ca, phosphate and 1,25(OH)2D3 levels down-regulate renal 1α-OHase expression. Serum 1,25(OH)2D3 levels are also regulated by the enzyme 25OHD3-24-hydroxylase (24OHase). Expression of 24OHase in the kidney is stimulated by 1,25(OH)2D3 and this enzyme converts 1,25(OH)2D3 into less active metabolites. These feedback mechanisms play an important role in the protection against hypercalcaemia and hyperphosphataemia(Reference Christakos, Dhawan and Benn10).
Extra-renal 1α-OHase expression and activity is modulated differently from renal 1α-OHase and is less sensitive to feedback regulation by 1,25(OH)2D3(Reference Hewison, Zehnder and Bland11). It is suggested that induction of extra-renal 1α-OHase involves regulatory pathways that differ from the renal, cyclic AMP-mediated pathway. For example, in osteoblasts, 1α-OHase expression and activity is not influenced by the levels of 1,25(OH)2D3, PTH and Ca like renal 1α-OHase. IL1β, an activator of NF-κB, stimulates both 1α-OHase expression and activity in osteoblasts(Reference van Driel, Koedam and Buurman4). In macrophages, immune signals such as TNFα and interferon-γ modulate 1α-OHase expression, while in vascular smooth muscle cells extra-renal 1α-OHase expression and activity is stimulated by the hormones PTH and oestrogen (Table 1)(Reference Somjen, Weisman and Kohen12, Reference Zehnder, Bland and Williams13). Regulators of 1α-OHase expression and activity in most extra-renal tissues and the functions of the extra-renally formed 1,25(OH)2D3 are still largely unknown. Some age-related effects on extra-renal 1α-OHase expression have been reported(Reference Somjen, Katzburg and Stern14). In an animal model, ageing resulted in decreased bone 1α-OHase expression(Reference Anderson, O'Loughlin and May15). Specific effects of ageing on the 1α-OHase expression and the activity in extra-renal tissues and health consequences of alterations in 1α-OHase expression and activity with ageing remain to be elucidated.
In recent years, several proteins have been discovered, which are important regulators of both Ca and phosphate homeostasis and the vitamin D endocrine system. These are fibroblast growth factor-23 (FGF-23) and klotho, a β-glucuronidase(Reference Chang, Hoefs and van der Kemp16, Reference Yamashita, Yoshioka and Itoh17). FGF-23 is involved in the regulation of renal phosphate excretion. FGF-23 inhibits expression of the renal sodium-phosphate transporter and thereby increases phosphate excretion(Reference Lanske and Razzaque18). In addition, FGF-23 decreases renal 1α-OHase expression, stimulates 24OHase expression and decreases both PTH mRNA expression and PTH secretion from the parathyroid gland(Reference Lanske and Razzaque18–Reference Shimada, Hasegawa and Yamazaki20). This leads to lower 1,25(OH)2D3 levels and thus decreases vitamin D-related effects on Ca and phosphate homeostasis. Klotho is involved in the regulation of renal Ca absorption and acts as a co-receptor or cofactor for other proteins such as FGF-23(Reference Urakawa, Yamazaki and Shimada21, Reference Nabeshima and Imura22). Klotho is capable of binding to various FGF receptors and enhances FGF-23 signalling. FGF-23 and klotho thus have important functions in regulating Ca and phosphate homeostasis and are important for skeletal health, both via effects on the vitamin D endocrine system and via direct, non-vitamin D-dependent effects.
Effects of ageing on vitamin D metabolism
Vitamin D deficiency is a worldwide problem(Reference Holick6). Although in Europe and the United States there has been strong attention on vitamin D in recent years and vitamin D-fortified food products are widely available, vitamin D deficiency is still very prevalent among older people(Reference Lanske and Razzaque23–Reference Yetley25). Mean serum 25OHD3 concentrations in The Netherlands in independent community-dwelling older people are about 30 nmol/l and in institutionalised older people about 20–25 nmol/l(Reference Chel, Wijnhoven and Smit26, Reference Lips27). Similar serum 25OHD3 levels among community-dwelling elderly have been reported in the United Kingdom and Germany(Reference Gallacher, McQuillian and Harkness28, Reference Hintzpeter, Mensink and Thierfelder29). Reports suggest that serum 25OHD3 levels in older people in the United States are higher than in Europe(Reference Chel, Wijnhoven and Smit26, Reference Holick, Siris and Binkley30, Reference Kuriacose and Olive31). This is most likely due to higher oral intake of vitamin D in the United States where vitamin D fortification of food is more prevalent than that in Europe(Reference Holden, Lemar and Exler24, Reference Calvo, Whiting and Barton32). However, even in the United States, >50 % of the community-dwelling elderly are reported to have serum 25OHD3 levels < 75 nmol/l and about 30 % of the elderly have levels < 50 nmol/l(Reference Holick, Siris and Binkley30).
The high prevalence of vitamin D deficiency in older people may have several causes. Cholecalciferol synthesis in the skin after sun exposure is less effective in old age because of a decline in cutaneous levels of 7-dehydrocholesterol(Reference MacLaughlin and Holick33). The level in a 70-year-old is only approximately 25 % of the 7-dehydrocholesterol level in young persons(Reference Holick and Chen34). This is worsened by the decreased exposure to sunlight with ageing due to immobility, lack of transport and social isolation(Reference Holick, Matsuoka and Wortsman35, Reference Adami, Giannini and Bianchi36). Another factor contributing to the increased risk of vitamin D deficiency is an increase in body fat with ageing. The increase in fat mass leads to a larger distribution volume for the fat-soluble 25OHD3, which decreases the bioavailability of 25OHD3. Consequently, an inverse association has been demonstrated between BMI and both serum levels 25OHD3 and 1,25(OH)2D3 and a positive association between BMI and PTH levels has been demonstrated(Reference Konradsen, Ag and Lindberg37, Reference Snijder, van Dam and Visser38).
An age-related decrease in 1,25(OH)2D3 levels has also been suggested but reports are conflicting(Reference Lau and Baylink39). When vitamin D levels are low, compensatory hyperparathyroidism increases renal conversion of 25OHD3 to 1,25(OH)2D3 and thereby maintains normal or even slightly elevated levels of this metabolite. As vitamin D deficiency worsens, 1,25(OH)2D3 formation is impaired due to a lack of substrate(Reference Lau and Baylink39). Additionally, several age-related effects have been reported that could lead to lower 1,25(OH)2D3 levels with ageing. First, renal function declines with age and this is accompanied with a decline in renal 1α-OHase activity and thus impaired conversion of 25OHD3 to 1,25(OH)2D3(Reference Dukas, Schacht and Mazor40). Second, levels of insulin-like growth factor 1, calcitonin and oestrogen, which stimulate 1α-OHase expression and activity, decrease with ageing(Reference Thorner and Nass41). Furthermore, 1,25(OH)2D3 metabolism may increase with ageing. In an animal model, an age-dependent increase in renal 24OHase expression was reported. This occurred predominantly in female animals, suggesting an effect of ovarian hormones(Reference Matkovits and Christakos42). Ovariectomy in these animals was indeed associated with up-regulation of 24OHase expression. Interestingly, the induction of 24OHase by 1,25(OH)2D3 may also be affected by ageing(Reference Armbrecht and Boltz43).
Effects of ageing on vitamin D action
The active metabolite 1,25(OH)2D3 exerts its function via the vitamin D receptor (VDR), a nuclear receptor. Upon binding of 1,25(OH)2D3, the VDR forms a heterodimer with the retinoid receptor and binds to a vitamin D-responsive element in the promoter region of a target gene. This influences transcription of vitamin D-responsive genes(Reference Lips1). In addition, the functions of the VDR are not limited to the binding to vitamin D-responsive element. The VDR has also been found to bind β-catenin, a key transcriptional factor in the Wnt signalling pathway(Reference Bikle44, Reference Shah, Islam and Dakshanamurthy45). This pathway has been implicated in a number of malignancies. By binding to β-catenin, the VDR blocks its transcriptional activity and so exerts antiproliferative properties. Besides genomic effects via the VDR, 1,25(OH)2D3 also exerts non-genomic effects via a membrane-bound plasma receptor or second messengers such as cyclic AMP. These are rapid effects that do not depend on gene transcription.
Almost all tissues and cells in the body express the VDR, including those not directly involved in the regulation of Ca homeostasis, enabling a broad range of effects. Extra-renal 1,25(OH)2D3 formation in various tissues implies that 1,25(OH)2D3 is also capable of exerting paracrine and autocrine effects in addition to the well-known endocrine effects. Among the paracrine and autocrine effects are regulation of cell proliferation, differentiation and apoptosis(Reference van Driel, Koedam and Buurman46).
Alterations in VDR expression leading to vitamin D resistance with ageing have received particular interest. With ageing, a decrease in VDR expression in bone, intestine and muscle tissue has been reported(Reference Duque, El Abdaimi and Macoritto47–Reference Bischoff-Ferrari, Borchers and Gudat49). Various factors are known to influence VDR expression. Oestrogen, growth hormones and 1,25(OH)2D3 are stimulators of VDR expression but serum levels decrease with ageing(Reference Welsh, Wietzke and Zinser50, Reference Klaus, Weber and Rodriguez51). On the other hand, TNFα has been shown to down-regulate VDR expression, while serum TNFα levels increase with ageing(Reference Andress52, Reference Fernandez-Martin, Kurian and Farmer53).
In addition to a decrease in VDR numbers, binding of 1,25(OH)2D3 to the VDR might also be decreased with ageing. A recent animal study, using competition VDR-binding assays with [3H]-1,25(OH)2D3, has reported a decrease in 1,25(OH)2D3 binding to the VDR with ageing in duodenal tissue(Reference Gonzalez Pardo, Boland and de Boland54). Whether this also occurs in human subjects is not known.
Parathyroid hormone and ageing
Like 25OHD3, PTH levels also exhibit seasonal variation with the highest PTH levels observed during the winter months(Reference Vecino-Vecino, Gratton and Kremer55). A (secondary) rise in PTH levels is generally observed with ageing with a prevalence varying from 20 to 60 %(Reference Bjorkman, Sorva and Tilvis56). The most important causes of this secondary rise with ageing are vitamin D deficiency and resistance, renal insufficiency and low dietary intake of Ca(Reference Perez, Picotto and Carpentieri57). PTH stimulates 1,25(OH)2D3 formation and mobilises Ca from bone in order to maintain normal serum Ca levels(Reference Lips1). Hyperparathyroidism not only negatively influences bone health but also is associated with sarcopaenia and falls as PTH stimulates muscle protein breakdown(Reference Visser, Deeg and Lips58). Furthermore, hyperparathyroidism has been related to cardiovascular events as PTH has been shown to promote vascular calcification(Reference Rashid, Bernheim and Green59). Recently, elevated PTH levels have been shown to be an independent predictor of impaired long-term survival prognosis in older people(Reference Bjorkman, Sorva and Tilvis56). High serum PTH levels ( ≥ 63 ng/l) were associated with significant increases in mortality (hazard ratio = 1·56, 95 % CI: 1·29, 1·88) and a 2·3-year reduction of median life expectancy in a cohort of older patients(Reference Bjorkman, Sorva and Tilvis56).
Calcium homeostasis and ageing
Ca homeostasis involves a coordinated control of Ca handling by the intestine, kidney and bone under the influence of primarily PTH and 1,25(OH)2D3. Ageing, vitamin D deficiency and vitamin D resistance all affect these processes negatively. The two main mechanisms for Ca (re)absorption are a transcellular (active) and a paracellular (passive) route. The transcellular route involves entry of Ca into the cell at the apical side of the cell via Ca channels, diffusion of Ca through the cytosol bound to calbindins and active extrusion of Ca across the basolateral membrane via a Ca pump or a Na/Ca exchanger(Reference Perez, Picotto and Carpentieri57). The epithelial Ca channels are members of the transient receptor potential (TRP) super family and more precisely, the vanilloid subfamily (TRPV). The TRPV5 channel is the major isoform in the kidney, while the TRPV6 channel is highly expressed in the intestine. The paracellular route involves diffusion of Ca via tight junctions between epithelial cells.
Ageing and intestinal calcium absorption
An age-related decrease in intestinal Ca absorption has long been recognised(Reference Bullamore, Wilkinson and Gallagher60). In the search for age-related factors that explain this decrease in absorption, attention has focused on TRPV6. A TRPV6 mouse knockout model illustrated the importance of TRPV6 for intestinal Ca absorption. In TRPV6 knockout mice, intestinal Ca absorption was decreased by 60 %(Reference Bianco, Peng and Takanaga61). Both in animal models and in human subjects, intestinal TRPV6 expression shows an age-dependent decline(Reference Walters, Balesaria and Chavele48). This is probably due to several effects as TRPV6 expression is regulated by 1,25(OH)2D3, oestrogen, PTH and dietary Ca intake(Reference Perez, Picotto and Carpentieri57). Recently, animal models have shed light on the importance of vitamin D metabolites for TRPV6 expression. Both in VDR and in 1α-OHase knockout mice, intestinal TRPV6 expression is strongly reduced, which impairs intestinal Ca absorption(Reference Hoenderop, van der Kemp and Urben62, Reference Song, Kato and Fleet63). In addition, the ability of vitamin D metabolites to stimulate intestinal TRPV6 expression also seems to decrease with ageing(Reference Armbrecht, Boltz and Kumar64).
The effects of ageing on TRPV6 expression differ among men and women. A recent study has reported that duodenal TRPV6 expression in both young and old men is strongly correlated with vitamin D status(Reference Walters, Balesaria and Chavele48). In women, however, TRPV6 expression decreased with ageing but no correlation was found with vitamin D status. In women, there was an age-dependent decline in VDR expression in the duodenal biopsies that was not found in men, which could account for the reduced vitamin D responsiveness and thus lower TRPV6 expression in women(Reference Walters, Balesaria and Chavele48). A possible explanation for decreased VDR expression could be decreased oestrogen levels with ageing. Oestrogen is important for vitamin D responsiveness as it stimulates both VDR and TRPV6 expressions(Reference Perez, Picotto and Carpentieri57). Although the strongest decline in intestinal Ca absorption is seen after the menopause due to decreasing serum levels of oestrogen, another late age-related decrease in intestinal Ca absorption, in addition to the decline that occurs at the menopause, has also been reported in women after the age of 75(Reference Nordin, Need and Morris65). This decrease in intestinal Ca absorption of nearly 30 % was independent of serum levels of 1,25(OH)2D3 and 25OHD3 and of renal function. The cause of this late decline in Ca absorption, which is most likely due to increased vitamin D resistance, remains to be clarified.
In men, the importance of the sex hormone testosterone for Ca absorption is not well known and remains to be studied. A stimulating effect of testosterone on TRPV6 expression has been suggested(Reference Scopacasa, Wishart and Horowitz66).
Ageing and renal calcium reabsorption
Less is known about the TRPV5 Ca channel. Like TRPV6, an age-related decrease in TRPV5 expression has been reported(Reference van Abel, Huybers and Hoenderop67). Expression of TRPV5 is mainly regulated by 1,25(OH)2D3, PTH and klotho(Reference Chang, Hoefs and van der Kemp16, Reference Hoenderop, Nilius and Bindels68). Klotho is important for TRPV5 expression as it cleaves a carbohydrate residue from the Ca channel TRPV5, which increases TRPV5 expression and activity by trapping it in the plasma membrane(Reference Chang, Hoefs and van der Kemp16). Expression of klotho itself is positively regulated by 1,25(OH)2D3 and oestrogen(Reference Lewin and Olgaard69). Several recent reports have demonstrated that klotho expression decreases with ageing(Reference Witkowski, Soroczynska-Cybula and Bryl70, Reference Duce, Podvin and Hollander71). In linking klotho expression to renal Ca absorption, it has been speculated that klotho deficiency may result in the down-regulation of TRPV5 expression and thus impairment of renal Ca reabsorption(Reference Nabeshima72). The importance of TRPV5 for renal Ca reabsorption has recently been demonstrated. TRPV5 knockout mice have severe hypercalciuria and decreased serum Ca levels(Reference Hoenderop, van Leeuwen and van der Eerden73). Klotho knockout mice exhibit both decreased renal TRPV5 expression and decreased renal Ca reabsorption(Reference Torres, Prie and Molina-Bletry74).
As serum Ca levels normally fluctuate between narrow margins, interplay between intestinal Ca absorption and renal reabsorption is required. A decrease in renal Ca reabsorptive capability is compensated for by an increase in intestinal absorption. A recent animal model has demonstrated that TRPV5 expression is an important determinant of TRPV6 expression. TRPV5 knockout mice have an increased intestinal TRPV6 expression and thus increased rate of intestinal Ca absorption(Reference van Abel, Huybers and Hoenderop67). In double TRPV5 and 1α-OHase knockout mice, the up-regulation of intestinal Ca transport was abolished suggesting that this is a vitamin D-dependent effect(Reference Renkema, Nijenhuis and van der Eerden75). In patients with idiopathic hypercalciuria, a disease state characterised by decreased renal Ca absorption and high urine levels of Ca, a compensatory increase in 1,25(OH)2D3 levels and intestinal Ca absorption is frequently observed(Reference Worcester and Coe76). The relevance of this interplay for maintaining Ca homeostasis in older people and effects of ageing remain to be studied.
Ageing and calbindins
Calbindins are cytosolic Ca-binding proteins. There are two major subclasses of calbindins: calbindin-D9k, which predominantly co-localises with TRPV6 in the small intestine, and calbindin-28k, which predominantly co-localises with TRPV5 in the kidney(Reference Perez, Picotto and Carpentieri57). Calbindins act to facilitate the diffusion of Ca through the cell interior towards the basolateral membrane. By buffering Ca, calbindins protect cells against toxic effects during states of high Ca influx. Anti-apoptotic effects of calbindins have been reported in different tissues such as neurons, osteoblasts and pancreatic β cells(Reference Christakos, Dhawan and Benn10). Calbindin expression decreases with ageing, which could contribute to decreased Ca (re)absorption with ageing due to impaired transcellular diffusion(Reference Matkovits and Christakos42). This is also influenced by vitamin D deficiency as vitamin D stimulates calbindin expression in both the intestine and the kidney(Reference Christakos, Dhawan and Benn10).
Other age-related effects on calcium absorption
PTH, besides stimulating intestinal Ca absorption via stimulation of renal 1α-OHase activity and thus 1,25(OH)2D3 formation and subsequently TRPV5 and TRPV6 expressions, also has direct effects on Ca absorption. The stimulation of duodenal Ca uptake by PTH has been demonstrated in an animal model(Reference Russo de Boland77). In rat enterocytes, PTH enhances Ca influx through activation of the voltage-gated apical Ca channels and the cyclic AMP second messenger system. Interestingly, in aged duodenal cells, PTH is more efficient in stimulating Ca absorption when compared with duodenal cells of young rats(Reference Massheimer, Picotto and Boland78). This is most likely due to alterations in signal transduction via the PTH receptor that occur with ageing. It has been speculated that this increased efficiency is a compensatory mechanism in older people in states of impaired vitamin D status(Reference Perez, Picotto and Carpentieri57).
Another determinant of Ca absorption is the bioavailability of dietary Ca itself. Low-Ca diets increase the efficiency of intestinal Ca absorption. The activities of all known genes involved in the transcellular pathway are enhanced by low-Ca diets, probably via activation of the vitamin D endocrine system(Reference Perez, Picotto and Carpentieri57).
What is vitamin D deficiency?
Measurement of serum 25OHD3 level is the best clinical indicator to assess vitamin D status. Serum 25OHD3 levels represent the combined contribution of both cutaneous synthesis and oral intake of the various dietary sources of vitamin D(Reference Zerwekh79). Levels of 1,25(OH)2D3 are less suitable to assess vitamin D status because even in a state of vitamin D deficiency 1,25(OH)2D3 levels can be normal or slightly elevated.
With the ever-increasing insights into the effects of vitamin D, optimal vitamin D status is becoming more difficult to define. Criteria and cut-off values for vitamin D deficiency have mostly been linked to the effects of PTH levels on bone turnover. Serum 25OHD3 levels are inversely associated with PTH levels until an inflection point is reached. At this point, PTH levels begin to level off. Estimates for the serum 25OHD3 concentration at which the PTH concentration becomes constant vary from 25 to 122 nmol/l(Reference Bjorkman, Sorva and Tilvis80, Reference Adami, Viapiana and Gatti81). This wide variation in estimates is due to inter-individual variation in Ca (re)absorption and vitamin D responsiveness, as previously discussed. Old people generally require higher serum 25OHD3 levels, and thus vitamin D intake, to suppress PTH levels when compared with younger individuals. The capacity of the different vitamin D metabolites to raise serum 25OHD3 levels is unaltered with ageing(Reference Vieth, Ladak and Walfish82).
When the effects on PTH levels and bone turnover are evaluated, serum 25OHD3 concentrations of >50 nmol/l are regarded by many as sufficient(Reference Lips83). However, when other health benefits of vitamin D are taken into account, including its non-calcaemic effects, serum 25OHD3 concentrations of >75 nmol/l are advised(Reference Heaney84). In addition, the process of extra-renal 1,25(OH)2D3 formation and autocrine and paracrine effects are most efficient when serum 25OHD3 levels are >75 nmol/l(Reference Holick6).
In many trials that study the effect of vitamin D supplementation, Ca intake is not measured, which complicates the comparison of individual trial results. Dietary Ca content has been shown to modulate the 25OHD3/PTH association(Reference Adami, Viapiana and Gatti81). As Ca intake is lower, higher 25OHD3 serum levels are required to normalise PTH concentrations. In part, this may also explain discordant results between intervention trials with vitamin D, as Ca intake differs among countries(Reference Boonen, Lips and Bouillon85, Reference Branca and Valtuena86).
Consequences of vitamin D deficiency and resistance
Vitamin D deficiency and resistance have important consequences for older people (Fig. 2). To illustrate its importance, vitamin D deficiency is associated with an increased risk for nursing home admission. The hazard ratio of nursing home admission after 6 years of follow-up for vitamin D-deficient individuals (25OHD3 < 25 nmol/l) in a large cohort of older people was 3·48 (1·39–8·75) when compared with individuals with a high serum 25OHD3 level. The hazard ratio for vitamin D-insufficient individuals (25OHD3 = 25–49 nmol/l) was 2·77 (1·17–6·55)(Reference Visser, Deeg and Puts87). The effects of vitamin D on bone, intestine and kidney, which are regarded as the classical target tissues, have been the subject of many studies for a long period of time. However, as the VDR is being found in increasingly more tissues, implications of vitamin D in many different disease states are being reported due to the effects of vitamin D outside these classical target tissues (Fig. 2). Detailed effects of vitamin D have been reported on cardiovascular health, immune system, neurological diseases and cancer. The discussion of the effects of vitamin D in these disease states is beyond the scope of the present paper, but excellent reviews have recently been published(Reference Hewison3, Reference McCann and Ames5, Reference Richart, Li and Staessen88, Reference Giovannucci89). Of note, recently adipose tissue has been shown to be a target tissue of vitamin D(Reference Duque and Troen90). With ageing, there is an accumulation of fat in bone marrow at the expense of osteoblastogenesis, contributing to the development of senile osteoporosis. Vitamin D has been shown to block adipogenesis by inhibiting the expression of PPARγ2, a critical transcription factor for adipogenesis in bone marrow(Reference Duque and Troen90).
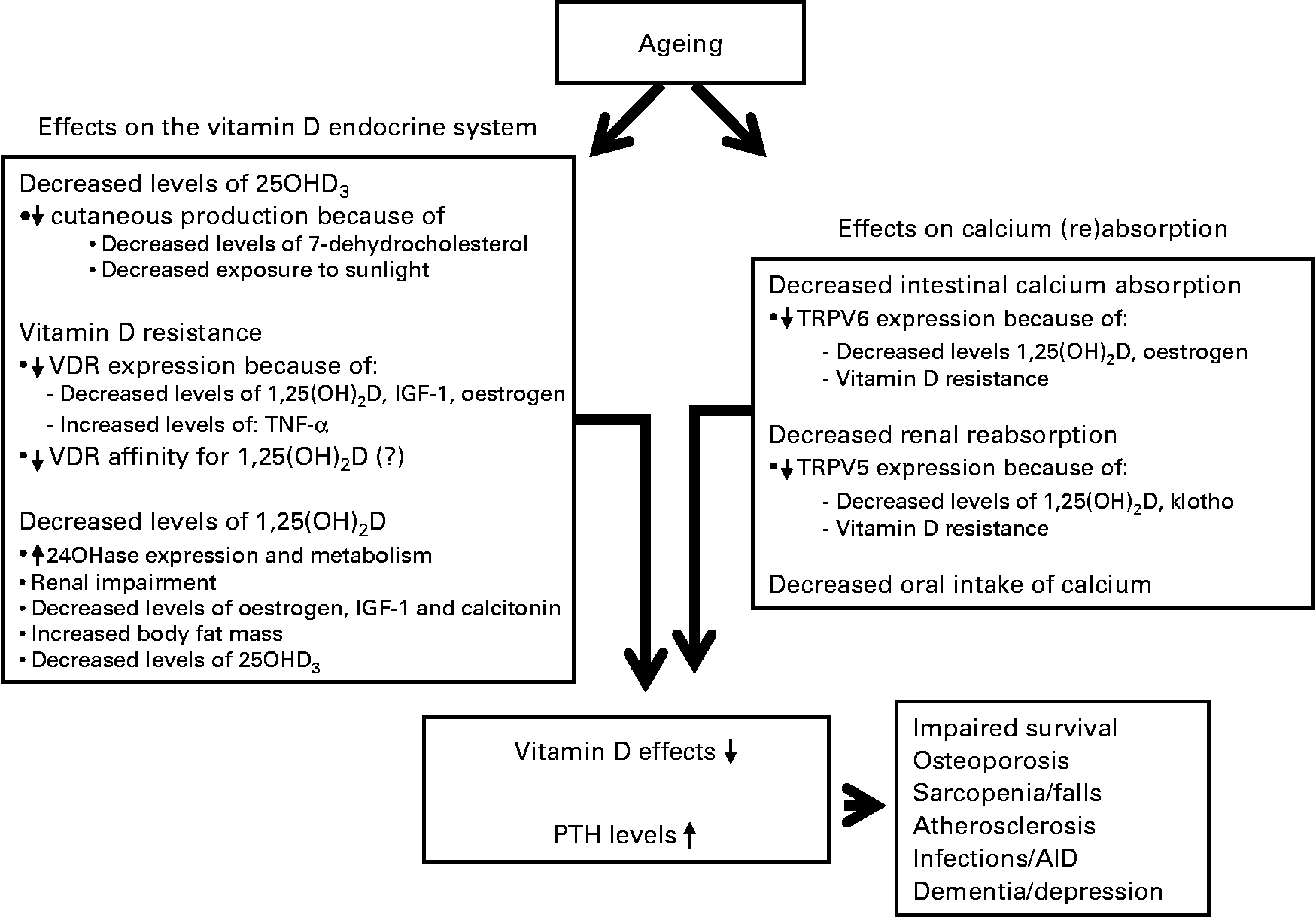
Fig. 2 Consequences of ageing on both vitamin D endocrine system and calcium absorption. 25OHD3, 25-hydroxyvitamin D3; VDR, vitamin D receptor; 1,25(OH)2D, 1,25-dihydroxyvitamin D; IGF, insulin-like growth factor; 24OHase, 24-hydroxylase; TRPV, transient receptor potential vanilloid; AID, auto-immune disorder.
In general, the advancing knowledge of the effects of vitamin D in all these tissues further strengthens the call for adequate treatment of vitamin D deficiency(Reference Kimball, Fuleihan Gel and Vieth91).
Treatment of vitamin D deficiency
Given the high prevalence of vitamin D deficiency in old age and the severe health consequences, a proactive approach from clinicians to case finding and adequate treatment of vitamin D deficiency is needed. Important considerations besides age are sex, BMI, skin colour, mobility, housing and dietary intake of both Ca and vitamin D(Reference Holick, Matsuoka and Wortsman35, Reference Bouillon, Verstuyf and Mathieu92, Reference Semba, Garrett and Johnson93). Giving individualised treatment advice is complicated by the fact that the ideal vitamin D level has not yet been defined and the treatment effect on, for example, secondary hyperparathyroidism is also dependent on the dietary intake of Ca, which shows regional differences. In general, mobile, Caucasian community-dwelling elderly, who have a varied diet, need vitamin D supplementation of 10–20 μg (400 IU–800 IU)/d to reach serum vitamin D levels of 50–75 nmol/l. Frail or institutionalised elderly on the other hand are suggested to need up to 50 μg (2000 IU)/d(Reference Heaney84, Reference Bischoff-Ferrari, Giovannucci and Willett94). The effectiveness of this high-dose vitamin D supplementation in raising serum 25OHD3 levels adequately has been demonstrated in several clinical trials(Reference Himmelstein, Clemens and Rubin95–Reference Stefikova, Chylova and Krivosikova97). However, robust evidence on the optimal dose of vitamin D supplementation in specific high-risk groups is still lacking. In a recent report by the Dutch Health Council(98), 20 μg (800 IU) daily is advised for high-risk groups, i.e., persons with osteoporosis, residents of care homes, women aged 50+ and men aged 70+ with dark skin colour or housebound individuals.
Oral supplementation is the most effective intervention to treat vitamin D deficiency. Ergocalciferol is equally as effective as cholecalciferol in raising serum 25OHD3 levels(Reference Holick, Biancuzzo and Chen99). Daily dosing is the most efficient interval to raise serum 25OHD3 concentrations when compared with weekly or monthly administration(Reference Chel, Wijnhoven and Smit26).
Although vitamin D supplementation therapy is generally regarded as safe, cases of iatrogenic and accidental overdose with cholecalciferol have been reported(Reference Blank, Scanlon and Sinks100, Reference Hathcock, Shao and Vieth101). Most safety data concerning the use of high-dose cholecalciferol supplementation come from observations in relatively young individuals. Few studies have used high-dose cholecalciferol supplementation for longer periods in frail, older patients. Frail old people, particularly the institutionalised, often have poor daily fluid intake, use diuretics and have less thirst sensation than younger persons.
The need for high-dose supplementation therapy on the one hand, and the increased risk of dehydration on the other hand, may potentially increase the risk of accidental hypercalcaemia in these patients.
Recently, concerns have risen regarding the possible negative health effects of vitamin D supplementation(Reference Richart, Li and Staessen88). The recent discovery of FGF-23 and klotho has given more insight into possible negative health effects of vitamin D supplementation and hypervitaminosis D. In animal studies, both FGF-23 and klotho knockout mice have increased expression of the enzyme 1α-OHase. These mice have increased serum levels of 1,25(OH)2D3, Ca and phosphate and have overall an identical phenotype. These knockout mice, despite their high levels of 1,25(OH)2D3, develop osteoporosis, vascular and soft tissue calcifications, muscle wasting, pulmonary emphysema and have a shortened lifespan(Reference Tsujikawa, Kurotaki and Fujimori102, Reference Shimada, Kakitani and Yamazaki103). Klotho knockout mice have very high levels of FGF-23 (about 2000-fold higher), but have no sign of phosphaturia, illustrating the importance of klotho for FGF-23 signalling(Reference Lanske and Razzaque18, Reference Urakawa, Yamazaki and Shimada21). Normalisation of vitamin D activity in both klotho and FGF-23 knockout mice by either feeding them a vitamin D-deficient diet or knockout of the 1α-OHase enzyme increased survival and rescued most of the phenotype, illustrating that these effects are indeed vitamin D related(Reference Tsujikawa, Kurotaki and Fujimori102, Reference Razzaque and Lanske104). Similar effects of hypervitaminosis D due to accidental overdose in human subjects have been reported(Reference Hathcock, Shao and Vieth101, Reference Jacobus, Holick and Shao105). Discontinuation of vitamin D supplementation in human subjects with hypervitaminosis D due to excessive intake of vitamin D resulted in normalisation of serum levels of 25OHD3, gradual recovery of bone density mineral and normalisation of the ratio of urinary Ca to creatinine(Reference Adams and Lee106). So, when a patient is suffering from osteoporosis, clinicians should also consider, although rare, the possibility of vitamin D overdose.
Conclusions
Vitamin D is a pleiotropic hormone. Besides the effects on classical tissues like bone and intestine, vitamin D has an effect on many more tissues. Effects of vitamin D metabolites can occur via endocrine, paracrine or autocrine mechanisms.
Ageing increases the risk of vitamin D deficiency and is associated with vitamin D resistance and less efficient intestinal Ca absorption and renal reabsorption. Vitamin D supplementation doses needed to treat vitamin D deficiency and secondary hyperparathyroidism vary considerably between individuals. This makes it necessary for clinicians to give tailored advice to patients when treating hypovitaminosis D, taking into account these age-related effects and other characteristics that influence vitamin D status and Ca homeostasis. All clinicians who frequently treat older patients should take a proactive approach to screening at-risk individuals for vitamin D deficiency, as this condition is still very prevalent. When treating patients for vitamin D deficiency, Ca intake should be assessed. Possible unwanted effects of long-term vitamin D supplementation and the effects of hypervitaminosis D should be studied in forthcoming trials.
Acknowledgements
The manuscript was written by C. O. and E. M. C., T. J. v. d. C. and M. E. T. M. provided a critical review of the sections on the effects of ageing and treatment of vitamin D deficiency. J. P. v. L. provided a critical review of the section on the actions of vitamin D and the section on Ca homeostasis. None of the authors had a personal or financial conflict of interest. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.





