Book contents
- Pediatric Head and Neck Pathology
- Pediatric Head and Neck Pathology
- Copyright page
- Contents
- Contributors
- Preface
- Acknowledgments
- Chapter 1 Diagnostic Methods and Specimen Handling Techniques in Pediatric Surgical Pathology
- Chapter 2 Soft Tissue Tumors and Reactive and Inflammatory Lesions of the Oral Cavity and Head and Neck
- Chapter 3 Cutaneous Tumors and Pseudotumors of the Head and Neck
- Chapter 4 Mucocutaneous Pigmented Lesions, Nevi and Melanoma
- Chapter 5 Vesiculo-Erosive and Ulcerative Lesions of Oral Cavity and Skin
- Chapter 6 Oral Epithelial Neoplasms
- Chapter 7 Odontogenic and Non-Odontogenic Cysts
- Chapter 8 Odontogenic Tumors
- Chapter 9 Non-Neoplastic Diseases of Salivary Glands
- Chapter 10 Benign Neoplasms of Salivary Glands
- Chapter 11 Malignant Neoplasm of Salivary Glands
- Chapter 12 Non-Neoplastic Disorders of the Nasal Cavity, Paranasal Sinuses and Nasopharynx
- Chapter 13 Benign Neoplasms of the Nasal Cavity, Paranasal Sinuses and Nasopharynx
- Chapter 14 Malignant Neoplasms of the Nasal Cavity, Paranasal Sinuses and Nasopharynx
- Chapter 15 Disorders of the Larynx and Hypopharynx
- Chapter 16 Neoplasms of Bone and Fibro-Osseous Lesions of the Craniofacial Skeleton
- Chapter 17 Disorders of the Ear
- Chapter 18 Disorders of the Thyroid Gland and Parathyroid Glands
- Chapter 19 Reactive and Malignant Diseases of Hematopoietic and Lymphoid Tissues
- Chapter 20 Developmental and Syndromic Disturbances of the Craniofacial Region
- Index
- References
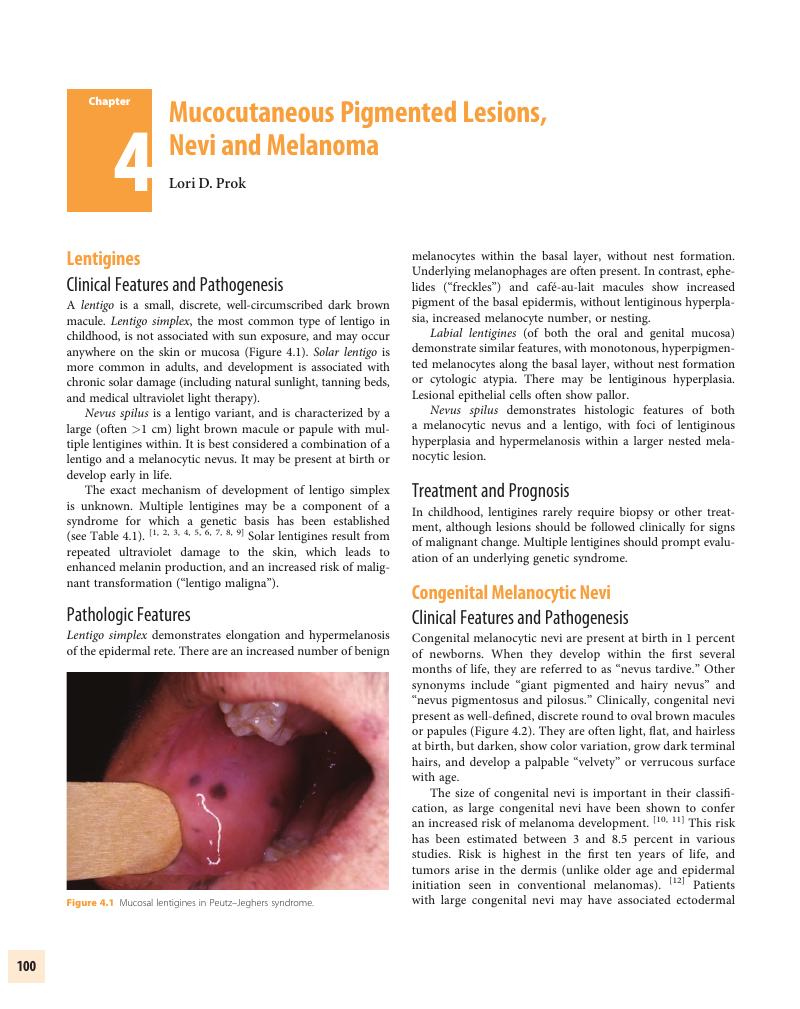
Chapter 4 - Mucocutaneous Pigmented Lesions, Nevi and Melanoma
Published online by Cambridge University Press: 26 June 2017
- Pediatric Head and Neck Pathology
- Pediatric Head and Neck Pathology
- Copyright page
- Contents
- Contributors
- Preface
- Acknowledgments
- Chapter 1 Diagnostic Methods and Specimen Handling Techniques in Pediatric Surgical Pathology
- Chapter 2 Soft Tissue Tumors and Reactive and Inflammatory Lesions of the Oral Cavity and Head and Neck
- Chapter 3 Cutaneous Tumors and Pseudotumors of the Head and Neck
- Chapter 4 Mucocutaneous Pigmented Lesions, Nevi and Melanoma
- Chapter 5 Vesiculo-Erosive and Ulcerative Lesions of Oral Cavity and Skin
- Chapter 6 Oral Epithelial Neoplasms
- Chapter 7 Odontogenic and Non-Odontogenic Cysts
- Chapter 8 Odontogenic Tumors
- Chapter 9 Non-Neoplastic Diseases of Salivary Glands
- Chapter 10 Benign Neoplasms of Salivary Glands
- Chapter 11 Malignant Neoplasm of Salivary Glands
- Chapter 12 Non-Neoplastic Disorders of the Nasal Cavity, Paranasal Sinuses and Nasopharynx
- Chapter 13 Benign Neoplasms of the Nasal Cavity, Paranasal Sinuses and Nasopharynx
- Chapter 14 Malignant Neoplasms of the Nasal Cavity, Paranasal Sinuses and Nasopharynx
- Chapter 15 Disorders of the Larynx and Hypopharynx
- Chapter 16 Neoplasms of Bone and Fibro-Osseous Lesions of the Craniofacial Skeleton
- Chapter 17 Disorders of the Ear
- Chapter 18 Disorders of the Thyroid Gland and Parathyroid Glands
- Chapter 19 Reactive and Malignant Diseases of Hematopoietic and Lymphoid Tissues
- Chapter 20 Developmental and Syndromic Disturbances of the Craniofacial Region
- Index
- References
Summary
- Type
- Chapter
- Information
- Pediatric Head and Neck Pathology , pp. 100 - 112Publisher: Cambridge University PressPrint publication year: 2016