Introduction
Dromedary camels are the most versatile livestock in the Arabian Peninsula: they produce milk, meat, wool, hides, and skins, and are used for riding, agricultural activities, racing, and many other cultural events (Saadeldin et al., Reference Saadeldin, Swelum, Alzahrani and Alowaimer2018). The propagation of camels under natural conditions is limited by their low reproductive performance, including the delayed onset of puberty, short breeding season, long calving interval, and high rate of pregnancy loss (Singh et al., Reference Singh, Mal, Gautam and Mukesh2019). Consequently, it is difficult and time-consuming to selectively increase camel populations for specific traits through natural breeding. Reproductive biotechnologies such as artificial insemination, in vitro fertilization, intracytoplasmic sperm injection, and multiple ovulation embryo transfer (MOET) have been used in many domesticated species to overcome low reproductive performance. In camels, MOET has been extensively used for several decades to improve production and performance by increasing the selection intensity of desired traits (McKinnon et al., Reference McKinnon, Tinson and Nation1994). The recent development of somatic cell nuclear transfer (SCNT) in camels has attracted much attention among different stakeholders (camel owners, breeders, competition organizers, researchers and veterinarians), as this approach has the potential to reproduce genetically identical elite camels in a relatively short period. Therefore, SCNT opens a new era for the commercialization of the camel cloning industry.
The success of the commercial application of camel cloning is largely dependent on the cost-effective acquisition of mature oocytes. In vitro-matured oocytes from abattoir samples could serve as a reliable source of low-cost matured oocytes for reproductive cloning (Khatir et al., Reference Khatir, Anouassi and Tibary2009). Superstimulation of the camel ovary with exogenous hormones followed by the collection of matured oocytes using the ultrasound-guided oocyte pick-up (OPU) method is a well established method in camels (Wani and Skidmore, Reference Wani and Skidmore2010; Ararooti et al., Reference Ararooti, Niasari-Naslaji, Razavi and Panahi2017). The first SCNT-derived dromedary camel was also produced using in vivo-matured oocytes. A subsequent study described the production of cloned camels using in vitro-matured oocytes (Wani et al., Reference Wani, Vettical and Hong2017). Although the pregnancy rate differs among species, the overall success rate of SCNT in camels is lower than in other mammals (Moulavi et al., Reference Moulavi, Asadi-Moghadam, Omidi, Yarmohammadi, Ozegovic, Rastegar and Hosseini2020). Accordingly, efforts will be needed to improve SCNT technology in camels.
The physiology of the reproductive tract varies greatly over the course of the reproductive cycle. The uterus remains receptive to a developing embryo for only a short period of time: on day 7 of the estrous cycle, the uterine endometrium is suitable for blastocysts, but maybe hostile to earlier-stage embryos, especially two- to four-cell embryos (Skidmore, Reference Skidmore, Skidmore and Adams2000; Anouassi and Tibary, Reference Anouassi and Tibary2013). Therefore, early-stage embryos should be transferred to the fallopian tube on day 2 of ovulation. A mismatch between the embryonic stage and the uterine environment may result in implantation failure. In large mammals such as cows and buffaloes, transcervical embryo transfer is the standard approach (Scherzer et al., Reference Scherzer, Fayrer-Hosken, Ray, Hurley and Heusner2008). Similarly, in camel, transcervical blastocyst transfer is widely practised in both MOET and SCNT programmes (McKinnon et al., Reference McKinnon, Tinson and Nation1994; Wani et al., Reference Wani, Wernery and Skidmore2010; Vettical et al., Reference Vettical, Hong and Wani2016). However, surgical embryo transfer is also possible in camel (Skidmore, Reference Skidmore, Skidmore and Adams2000). In humans, a comparison between early-stage and blastocyst embryos originating from in vitro fertilization revealed that higher live birth rates were associated with blastocyst-stage embryo transfer (Shahrokh Tehraninejad et al., Reference Shahrokh Tehraninejad, Azimi Nekoo, Ghaffari, Hafezi, Karimian and Arabipoor2015; Glujovsky et al., Reference Glujovsky, Farquhar, Quinteiro Retamar, Alvarez Sedo and Blake2016). However, this kind of study has not been extensively performed on SCNT-derived embryos in animals, and no studies have been conducted on the influence of embryo stage derived from in vitro-matured oocytes on implantation and pregnancy rate in camels. Therefore, in this study, we evaluated the comparative efficiency of early-stage and blastocyst-stage SCNT-derived embryos produced from in vitro-matured oocytes in yielding live births in camels.
Materials and methods
Chemicals
All chemicals and reagents were purchased from Sigma (St. Louis, MO, USA) unless otherwise stated.
Selection and management of recipients
Camels in good health and without any abnormalities in the reproductive tract were selected and used as recipients. They were fed appropriate nutrients daily and given water ad libitum. In total, 23 camels aged from 4 to 7 years, weighing 400–450 kg, were used in this study. The recipients were treated with a single intramuscular injection of 1500 IU pregnant mare serum gonadotropin (PMSG) and 100 µg of cloprostenol on day 0. On day 9, the recipients were injected with 100 µg gonadorelin acetate (Vétoquinol, Paris, France) to promote ovulation and corpus luteum (CL) formation.
Oocyte collection from abattoir ovaries
Ovaries were collected from a local abattoir and transported to the laboratory in lukewarm 0.9% saline solution. Cumulus–oocytes complexes (COCs) were aspirated from antral follicles 2–6 mm in diameter through an 18-gauge hypodermic needle attached to a 10 ml disposable syringe. COCs with homogenous cytoplasm and having at least three layers of compact cumulus cells were selected and washed three times in Dulbecco’s phosphate-buffered saline (DPBS; Welgene, Gyeongsan, Korea) supplemented with 5 mg/ml bovine serum albumin (BSA; Thermo Fisher Scientific, Waltham, MA, USA) and 1% antibiotic–antimycotic mix (Thermo Fisher Scientific). The collected COCs were cultured in a commercial in vitro maturation (IVM) medium (IVF Bioscience, Falmouth, UK) for 42 h at 38°C in a humidified atmosphere containing 5% CO2.
Establishment of a skin fibroblast cell line
Fibroblast cell lines were established from the ear skin of an elite camel; samples were obtained as previously described with minor modifications (Wani et al., Reference Wani, Wernery and Skidmore2010). Briefly, tissues were washed three times with DPBS supplemented with 1% antibiotic–antimycotic. After that, samples were minced into small pieces with a surgical blade and digested at 38°C in a humidified atmosphere with 5% CO2 for 2 h in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific) supplemented with 0.1% collagenase type II (Thermo Fisher Scientific). The dispersed cells were washed with DPBS by centrifugation at 300 g for 5 min and filtered through a 40-µm nylon strainer (Falcon, Franklin, NJ, USA). The cell pellets were cultured at 38°C in a humidified atmosphere with 5% CO2 in DMEM supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific), 1% nonessential amino acids (Thermo Fisher Scientific), 1% antibiotic–antimycotic (Thermo Fisher Scientific), and 0.1% β-mercaptoethanol (Thermo Fisher Scientific). The culture medium was changed every 2 days until confluency reached 80%, and then the cells were passaged using 0.25% trypsin–EDTA solution.
Somatic cell nuclear transfer
SCNT was performed as previously described with minor modifications (Wani et al., Reference Wani, Wernery and Skidmore2010). In brief, oocytes were denuded by gentle pipetting with 0.1% hyaluronidase. Denuded metaphase II oocytes were stained with 5 μg/ml bisbenzimide for 3 min. The oocytes were enucleated by aspiration, and a single fibroblast cell was microinjected into the perivitelline space of each oocyte. Next, these oocyte couplets were fused in fusion medium composed of 0.26 M mannitol, 0.1 mM MgSO4, 0.5 mM HEPES, and 0.05% (w/v) BSA with two direct current (DC) pulses of 1.8 kV/cm for 15 μs using a BTX Electro Cell Manipulator (BTX Inc., San Diego, CA, USA). The reconstructed oocytes were activated using 5 μM ionomycin for 3 min, followed by incubation with 2.0 mM 6-dimethylaminopurine (6-DMAP) in the commercial embryo culture medium BO-IVC (IVF Bioscience, Falmouth, UK) at 39°C for 4 h in a humidified incubator with 5% CO2.
Experimental design
Following activation, reconstructed oocytes were cultured in BO-IVC. Groups of six to eight oocytes were cultured in 30 μl oil-covered droplets at 38°C in a humidified atmosphere with 5% CO2 and 5% O2.
In group A, 380 in vitro-matured oocytes were reconstructed and 291 fused oocytes were cultured for 2 days; the early-stage embryos were surgically transferred to the recipients 72 h after gonadorelin acetate injection (2 days post-ovulation). For surgical embryo transfer, recipients were placed in a padded crush and sedated by intravenous injection of 100 mg of xylazine (Ceva, Libourne, France). An inverted ‘L’ block was infiltrated on the left flank of the abdomen in front of the anterior crest of the ilium using 2% lidocaine. After anaesthesia, the fimbriae of the left ovary were exposed by incision. Embryos were loaded into a catheter (Sherwood Medical, St. Louis, MO, USA) with 4 µl of transfer medium (IVF Bioscience, Falmouth, UK) and gently transferred deeply into the oviduct.
In group B, 326 in vitro-matured oocytes were reconstructed and 239 fused oocytes were cultured for 7 days. Transcervical blastocyst transfer was performed in synchronized females (day 7 of ovulation).
Pregnancy diagnosis
Pregnancy was detected by evaluating high levels of serum progesterone on 21 (early embryos) or 16 (blastocysts) days after embryo transfer, measured by chemiluminescence immunoassay (Roche, Basel, Switzerland). Animals exhibiting an initial rise in serum progesterone level to >1 ng/ml were considered to be pregnant. Pregnancies were confirmed using real-time ultrasonography on 30 and 90 days after embryo transfer.
Microsatellite analysis
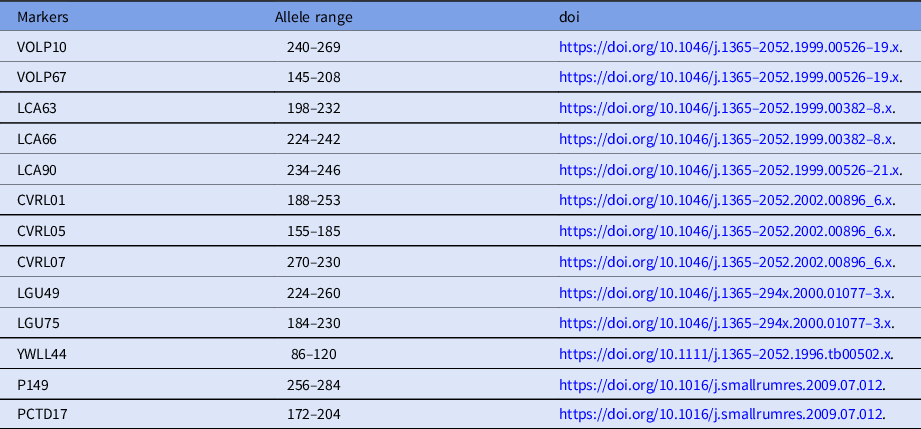
To confirm the reliability of the cloned calves from the donor cells, microsatellite analysis was carried out using 13 specific loci for Camelus dromedarius (Table 1). DNA was isolated from individual donor cells, venous blood of cloned calves, and recipients using the Qiagen DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany).
Table 1. Characteristics of 13 microsatellite loci for Camelus dromedarius
Statistical analysis
All data analyses were performed using SPSS (version 15; SPSS Inc., Chicago, IL, USA). To analyze differences in the development of embryos and the average number of transferred embryos between the groups, Student’s t-test was performed. When comparing the pregnancy rates, Pearson chi-squared test and Fisher’s exact test were conducted. Data were represented as means ± standard error (SE), and P-values less than 0.05 were considered to be statistically significant.
Results
In vitro maturation of camel oocytes
Data regarding in vitro maturation of camel oocytes collected from abattoir samples are presented in Table 2. After 42 h of culture, the cumulus–oocytes complexes were denuded in 1% hyaluronidase by gentle pipetting and graded under a stereomicroscope as mature, immature or abnormal. The maturation (metaphase II) rates were 70.48% and 68.41% for groups A and B, respectively. The percentages of immature oocytes were 27.00% and 29.01% for groups A and B, respectively.
Table 2. In vitro maturation of camel oocytes derived from abattoir samples in the two experimental groups
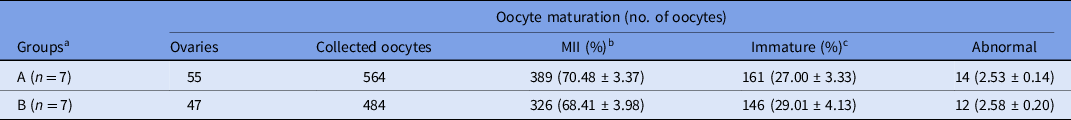
a Groups A and B were defined based on the use of SCNT-derived embryos. Percentage (%) is the average of seven replicates.
b MII = metaphase II oocytes.
c Immature = germinal vesicle, germinal vesicle breakdown and metaphase I oocytes.
Values in different columns did not differ at P < 0.05.
Developmental competence of SCNT-derived camel embryos
Data regarding in vitro development of camel embryos produced by SCNT using in vitro-matured oocytes are presented in Table 3. In group A, 389 oocytes were reconstructed and 291 SCNT-derived embryos (fusion rate: 73.46%) were cultured for 2 days; in group B, 326 oocytes were reconstructed and 239 SCNT-derived embryos (fusion rate: 73.72%) were cultured for 7 days. The differences in fusion rate were not statistically significant. Embryos developed in group A were transferred surgically to the recipient on day 2, and embryos developed in group B were cultured for 7 days. The blastocyst production rate in group B was 21.77%. Transcervical blastocyst transfer was performed in the recipients.
Table 3. In vitro development of camel embryos derived by somatic cell nuclear transfer using in vitro-matured oocytes
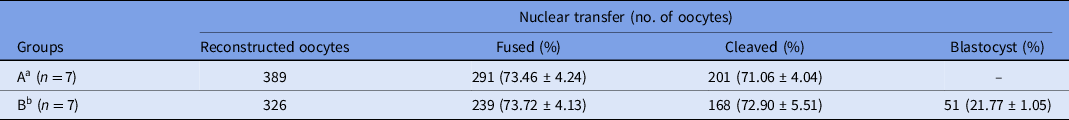
a Group A was subjected to in vitro culture (IVC) for 2 days and the embryos were surgically transferred into recipients.
b Group B was subjected to in vitro culture (IVC) for 7 days and blastocysts were transvaginally transferred into recipients.
Values in different columns did not differ at P < 0.05.
Efficiency of pregnancy rate and identification in cloned camel
The pregnancy rates and percentage of live birth following early-stage embryo or blastocyst transfer are shown in Table 4. In total, 71 early-stage embryos were transferred into 28 recipients and 47 blastocysts were transferred into 26 recipients. Clinical pregnancy rates based on P4 were 17.86% and 11.54% for early-stage and blastocyst transfer, respectively. On day 30, one pregnancy loss was observed in both groups.
Table 4. Pregnancy and live birth rates following early-stage embryo and blastocyst transfer
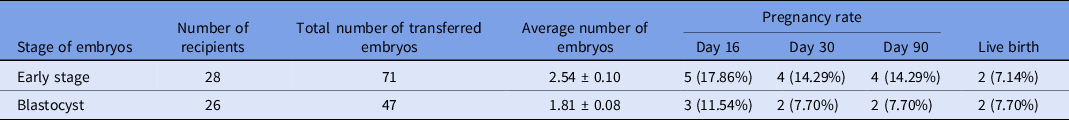
Values in different columns did not differ at P < 0.05.
Subsequently, out of four pregnant females in group A, two aborted at 17 and 24 weeks of pregnancy, whereas two remained pregnant and gave birth to normal and healthy offspring. In group B, no pregnancy loss was observed after day 30, and both pregnant females gave birth to normal healthy offspring.
Microsatellite analysis of 13 camel loci revealed that the SCNT-derived offspring were identical to their somatic cell donor (Table 5).
Table 5. Microsatellite analysis a of Camelus dromedaries
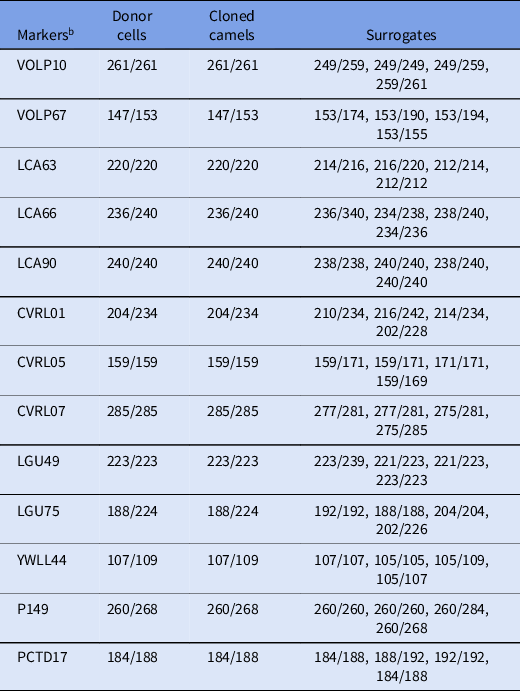
a Microsatellite analysis was performed on genomic DNA from cloned offspring as well as surrogate and donor cells.
b The values of all markers were confirmed to be identical in all cloned offspring.
Values represent base pairs of the amplified microsatellite DNA markers in each sample.
Discussion
In this study, we used in vitro-matured oocytes to produce camel embryos using SCNT and then compared the influence of early-stage embryo versus blastocyst transfer on pregnancy and live birth rates. Because in vitro-matured oocytes are a more cost-effective material source for SCNT in camels, we evaluated the success of cloning from a single source, which yielded four cloned camels, two from early-stage embryo transfer and two from blastocyst transfer. Early pregnancy rates were significantly higher for early-stage embryo transfer than blastocyst transfer (17.86% and 11.54%, respectively), and the rates of live birth per pregnancy were similar (7.14% and 7.70%, respectively).
The availability of mature oocytes is the main limiting factor for the large-scale adoption of reproductive biotechnologies. Aspiration of COC from ovaries collected from abattoirs is the easiest and most economic source of oocytes (Moawad et al., Reference Moawad, Ghoneim, Darwish, Badr, El-Badry and El-Wishy2020). Follicular aspiration is the most suitable method for COC retrieval in dromedary camels (Jain et al., Reference Jain, Das, Solanki and Tripathi1995; Kumar et al., Reference Kumar, Solanki, Jindal, Tripathi and Jain1997). This is due in part to the fact that camel ovarian follicles protrude from the surface of ovaries as spherical, discrete, thick-walled structures (El-Wishy and Hemeida, Reference El-Wishy and Hemeida1984; Arthur et al., Reference Arthur, Al-Rahim and Al-Hindi1985). Our oocyte maturation rate (metaphase II oocytes) was 68–70%, whereas Yaqoob et al. (Reference Yaqoob, Saadeldin, Swelum and Alowaimer2017) reported 60%, Wani and Nowshari (Reference Wani and Nowshari2005) reported 52%, and Fathi et al. (Reference Fathi, Moawad and Badr2018) reported 55%. Although the maturation condition and duration of the culture period differed in those studies, at least 50% of oocytes selected from abattoir samples reached the metaphase II stage. We observed that in vitro blastocysts developed at a rate of 21.77% from in vitro-matured oocytes following SCNT. In a previous study, Moulavi et al. (Reference Moulavi, Asadi-Moghadam, Omidi, Yarmohammadi, Ozegovic, Rastegar and Hosseini2020) reported that blastocysts developed at a rate of 14.1% using in vitro-matured oocytes.
Embryo transfer location and proper matching of embryo stage with transfer location in recipients are crucial for the establishment and maintenance of pregnancy and live births (Skidmore, Reference Skidmore, Skidmore and Adams2000). Following natural conception, camel embryos reach the uterus at 6–6.5 days post-ovulation at the blastocyst stage (Skidmore, Reference Skidmore, Skidmore and Adams2000; Anouassi and Tibary, Reference Anouassi and Tibary2013). In this study, we transferred early-stage embryos to the fallopian tube on the second day of ovulation (the third day of intravenous injection of gonadorelin acetate), as the uterine endometrium does not provide an appropriate physiological environment for early-stage embryos (Skidmore, Reference Skidmore, Skidmore and Adams2000). Accordingly, blastocyst-stage embryos were transferred to the uterine horns on the seventh day of ovulation, according to the physiological schedule of embryo development in this species.
Embryos produced in vitro using SCNT can be transferred at the early stage or blastocyst stage. Transvaginal blastocyst-stage embryo transfer is the preferred method in large animals, due to the relative ease of access to the reproductive tract (Scherzer et al., Reference Scherzer, Fayrer-Hosken, Ray, Hurley and Heusner2008). To date, we are the only group to have produced cloned camels using a method other than transvaginal embryo transfer. Skidmore (Reference Skidmore, Skidmore and Adams2000) reported that early-stage embryos can be transferred surgically into the fallopian tube in camel; however, no live births have been reported following the surgical transfer of embryos.
Blastocyst transfer has advantages when compared with early-stage embryo transfer because it allows for self-selection of embryos: embryos that develop into blastocysts in vitro are more likely to be viable after transfer and result in a viable pregnancy (Shahrokh Tehraninejad et al., Reference Shahrokh Tehraninejad, Azimi Nekoo, Ghaffari, Hafezi, Karimian and Arabipoor2015). The embryo culture period largely depends on the in vitro culture (IVC) system; culture of embryos up to the blastocyst stage is preferred if the IVC system is sufficient to support their routine development. Suboptimal culture conditions may lead to arrested embryonic development or low-quality blastocysts that fail to develop or maintain pregnancies. Camel IVC is less well defined than that of other domestic animals (Saadeldin et al., Reference Saadeldin, Swelum and Alowaimer2019). We observed 21.77% blastocyst formation rates. Among others, this could be an indication of suboptimal culture conditions in camels. Fernández-Gonzalez et al. (Reference Fernández-Gonzalez, Ramirez, Bilbao, De Fonseca and Gutiérrez-Adán2007) reported that suboptimal culture conditions arrested the development of embryos; in addition, epigenetic changes may occur in developing embryos, resulting in pregnancy failure and developmental abnormalities. In this study, out of 168 cleaved embryos, 51 developed blastocysts (21.77%). Transfer of early-stage embryos decreases the exposure time to in vitro culture and is thought to minimize the detrimental effects originating from IVC. However, early-stage embryo transfer in camels is challenging and requires laparotomic surgery (Skidmore, Reference Skidmore, Skidmore and Adams2000). The influence of embryo stage on pregnancy rate in camels has not previously been reported. Therefore, we conducted this comparative study with the hypothesis that the potential benefit of early-stage embryo transfer would supersede the difficulties associated with the transfer method. However, we observed no significant differences in the pregnancy or live birth rates between the blastocyst and early-stage embryo transfer.
Live births were the major metric for this study, as reproductive efficiency in camels is greatly reduced by early- and late-term pregnancy loss. Anouassi and Tibary (Reference Anouassi and Tibary2013) reported a rate of embryonic death up to 35% in dromedary camels. Early pregnancy diagnosis is routinely based on P4 levels and may often be misleading; therefore, the use of ultrasonographic examinations to clinically determine pregnancy is warranted. In this study, we observed a 60% pregnancy loss (two live births out of five clinically pregnant camels) in the early-stage embryo transfer group versus a 33% pregnancy loss (two live births out of three clinically pregnant camels) in the blastocyst transfer group. It is difficult to establish the causes of embryonic death in camels or to decrease embryonic loss without further knowledge. Several factors may influence the likelihood of camel embryo loss, including quality of the embryo or CL, P4 insufficiency, uterine environment, and physiological conditions. Multiple variables must be optimized to overcome the limitations of camel cloning and embryo transfer, and further developments will undoubtedly shed light on the unique physiological traits of this species.
In conclusion, in vitro-matured oocytes can be efficiently used for SCNT in camels, and both early-stage embryos and blastocysts produced by SCNT using in vitro-matured oocytes can yield live offspring. Pregnancy and birth rates were similar in both groups. Considering the recipients’ well-being and the ease of the transfer procedure, we recommend transvaginal blastocyst transfer.
Acknowledgements
The authors acknowledge the service of Camel Biotechnology Center (CBC), Presidential Camels and Camel Racing Affairs, Al-Ain, UAE, for confirming the parentage of clones by short tandem repeat (STR) analysis.
Financial support
This project was supported by the Patronage of H.H. Sheikh Mansour bin Zayed Al Nahyan, Deputy Prime Minister of the UAE and the Minister of Presidential Affairs, UAE. We acknowledge his support and inspiration in the initiation and mentoring of this project, without whom this project would not have been possible.
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical standards
All animal procedures in this study were conducted in accordance with animal study guidelines reviewed and approved by the Ethics Committee of the Management of Scientific Center for Presidential Camels (Permit Number: PC4.1.5). The guideline complies with the ARRIVE guidelines and was performed in accordance with the UK Animals (Scientific Procedure) Act, 1986 and associated guidelines (EU Directive 2010/63/EU).







