INTRODUCTION
In the days of Sigmund Freud, obsessive-compulsive disorder (OCD) was deemed the prototype of a neurotic disorder and was regarded as both amenable to understanding and psychological treatment (Freud, Strachey, Freud, Strachey, & Tyson, Reference Freud, Strachey, Freud, Strachey and Tyson1909). Psychoanalytic models were the starting point of contemporary OCD research; these assumed an unresolved conflict in OCD between the urges from the so-called “id” that collide with the hypermoral demands of the so-called “superego” (Freud, Reference Freud1963; Moritz, Kempke, Luyten, Randjbar, & Jelinek, Reference Moritz, Kempke, Luyten, Randjbar and Jelinek2011). The merits of psychological models of OCD are undisputed to this day. Yet, cognitive behavioral therapy has replaced psychoanalysis as the primary (evidence-based) treatment for OCD (Gava et al., Reference Gava, Barbui, Aguglia, Carlino, Churchill, De Vanna and McGuire2007; Skapinakis et al., Reference Skapinakis, Caldwell, Hollingworth, Bryden, Fineberg, Salkovskis and Lewis2016).
Paralleling trends in other fields of psychopathology research (e.g., depression, schizophrenia), the interest in biological models of OCD is on the rise (Abramovitch, Abramowitz, & Mittelman, Reference Abramovitch, Abramowitz and Mittelman2013; Hu et al., Reference Hu, Du, Chen, Li, Zhou, Zhang and Gong2017; Peng et al., Reference Peng, Lui, Cheung, Jin, Miao, Jing and Chan2012; Rotge et al., Reference Rotge, Langbour, Guehl, Bioulac, Jaafari, Allard and Burbaud2010). This type of research is stimulated by studies indicating that OCD is associated with neuropsychological deficits across several domains (Abramovitch et al., Reference Abramovitch, Abramowitz and Mittelman2013; Benzina, Mallet, Burguière, N’Diaye, & Pelissolo, Reference Benzina, Mallet, Burguière, N’Diaye and Pelissolo2016; Kuelz, Hohagen, & Voderholzer, Reference Kuelz, Hohagen and Voderholzer2004; Shin, Lee, Kim, & Kwon, Reference Shin, Lee, Kim and Kwon2014; Snyder, Kaiser, Warren, & Heller, Reference Snyder, Kaiser, Warren and Heller2015; Tallis, Reference Tallis1997), particularly nonverbal memory and executive functioning, allegedly stemming from brain deficits in areas such as the (orbito-)frontal cortex (Hu et al., Reference Hu, Du, Chen, Li, Zhou, Zhang and Gong2017; Pauls, Abramovitch, Rauch, & Geller, Reference Pauls, Abramovitch, Rauch and Geller2014; Peng et al., Reference Peng, Lui, Cheung, Jin, Miao, Jing and Chan2012; Piras et al., Reference Piras, Piras, Chiapponi, Girardi, Caltagirone and Spalletta2015; Rotge et al., Reference Rotge, Langbour, Guehl, Bioulac, Jaafari, Allard and Burbaud2010).
Several researchers advocate the idea that neurocognitive deficits may represent endophenotypes of the disorder (Chamberlain, Blackwell, Fineberg, Robbins, & Sahakian, Reference Chamberlain, Blackwell, Fineberg, Robbins and Sahakian2005; Robbins et al., Reference Robbins, Gillan, Smith, de Wit and Ersche2012; Snyder et al., Reference Snyder, Kaiser, Warren and Heller2015). The account that OCD is an entirely psychological disorder has accordingly crumbled. This inference is, however, not completely undisputed in view of some recent findings indicating that contextual factors such as poor motivation (Moritz, Hauschildt, Saathoff, & Jelinek, Reference Moritz, Hauschildt, Saathoff and Jelinek2017; Moritz, Hottenrott, Jelinek, Brooks, & Scheurich, Reference Moritz, Hottenrott, Jelinek, Brooks and Scheurich2012), threatened morality (Kalanthroff, Aslan, & Dar, Reference Kalanthroff, Aslan and Dar2017), comorbid disorders (Basso, Bornstein, Carona, & Morton, Reference Basso, Bornstein, Carona and Morton2001; Moritz et al., Reference Moritz, Birkner, Kloss, Jacobsen, Fricke, Böthern and Hand2001), and interference with symptoms (Abramovitch, Dar, Hermesh, & Schweiger, Reference Abramovitch, Dar, Hermesh and Schweiger2012) contribute to poorer test performance in patients with OCD (see below). Of interest, similar moderators are being discussed for other disorders, for example schizophrenia (Fervaha et al., Reference Fervaha, Zakzanis, Foussias, Graff-Guerrero, Agid and Remington2014; Krkovic, Moritz, & Lincoln, Reference Krkovic, Moritz and Lincoln2017; Moritz et al., Reference Moritz, Klein, Desler, Lill, Gallinat and Schneider2017).
Psychological and biological models are not mutually exclusive. Clearly, every cognition and emotion has a biological substrate, and for more than 25 years we have known that psychotherapy in OCD impacts brain functioning (Baxter, Reference Baxter1992). Freud himself was interested in the physiological correlates of psychological processes (Centonze, Siracusano, Calabresi, & Bernardi, Reference Centonze, Siracusano, Calabresi and Bernardi2004; Freud, Reference Freud1953). Yet, contemporary research suggests widespread structural abnormalities within the frontal-striatal network in OCD (Hu et al., Reference Hu, Du, Chen, Li, Zhou, Zhang and Gong2017; Peng et al., Reference Peng, Lui, Cheung, Jin, Miao, Jing and Chan2012) rather than subtle, temporal, and changeable biological alterations.
OCD is associated with both stigma (Fennell & Liberato, Reference Fennell and Liberato2007; Warman, Phalen, & Martin, Reference Warman, Phalen and Martin2015) and self-stigma, which is one of the major reasons for treatment abstinence in the disorder (García-Soriano, Rufer, Delsignore, & Weidt, Reference García-Soriano, Rufer, Delsignore and Weidt2014; Marques et al., Reference Marques, LeBlanc, Wegarden, Timpano, Jenike and Wilhelm2010). Self-stigma is rooted in the deep worry of many individuals with OCD that they are dangerous to loved ones or the public. Stigma and self-stigma are pronounced in patients with taboo thoughts (e.g., thoughts about harming other people; Glazier, Wetterneck, Singh, & Williams, Reference Glazier, Wetterneck, Singh and Williams2015; McCarty, Guzick, Swan, & McNamara, Reference McCarty, Guzick, Swan and McNamara2017), fueling their fears about involuntary hospitalization (Glazier et al., Reference Glazier, Wetterneck, Singh and Williams2015). Fear of resentment by co-workers and by potential partners was endorsed frequently in one of the earliest studies on this topic (Stengler-Wenzke, Beck, Holzinger, & Angermeyer, Reference Stengler-Wenzke, Beck, Holzinger and Angermeyer2004).
Anti-stigma campaigns fostering awareness and understanding of psychological disorders often convey that those with mental disorders are not dangerous and that the sufferers have a biological vulnerability (Briffault, Morvan, & Roscoät, Reference Briffault, Morvan and Roscoät2010; Lincoln, Arens, Berger, & Rief, Reference Lincoln, Arens, Berger and Rief2008; Wiesjahn, Jung, Kremser, Rief, & Lincoln, Reference Wiesjahn, Jung, Kremser, Rief and Lincoln2016) and thus cannot be held fully responsible for their occasionally disturbing ideas and actions. Biological models, such as the idea that OCD reflects a disturbance in the basal ganglia or orbito-frontal cortex (for one of the most influential models, see Saxena, Brody, Schwartz, & Baxter, Reference Saxena, Brody, Schwartz and Baxter1998), may decrease the burden of shame in some patients by reducing their self-blame (as captured by the famous quote “It is not me but my OCD”) (Angermeyer, Holzinger, Carta, & Schomerus, Reference Angermeyer, Holzinger, Carta and Schomerus2011) but may in turn create negative consequences with regard to stigma and self-stigma. Indeed, studies show that biogenetic models increase endorsement of the stereotype that people with psychological problems are dangerous (Kvaale, Haslam, & Gottdiener, Reference Kvaale, Haslam and Gottdiener2013). Another negative consequence of biological models is fatalism regarding treatment. In a recent study (Moritz et al., Reference Moritz, Stepulovs, Schröder, Hottenrott, Meyer and Hauschildt2016), 36% of individuals with OCD endorsed that having a brain disorder likely means that psychotherapy is not effective. To conclude, biogenetic models may harm patients’ self-esteem and compromise treatment outcomes. This is particularly troublesome if the studies these models rely on exaggerate the real degree of deficits and/or only apply to a subgroup of patients.
Our study tested the hypothesis that stereotype threat contributes to poor neuropsychological performance in people with OCD. Stereotype threat is a well-established (social) psychological effect. It denotes the phenomenon that performance is compromised when an individual is confronted with a devaluing stereotype or stigma-related information (Schmader, Johns, & Forbes, Reference Schmader, Johns and Forbes2008; Steele & Aronson, Reference Steele and Aronson1995). The effect is ubiquitous and has been demonstrated across various groups, including people of different races, genders, and ages (Derks, Inzlicht, & Kang, Reference Derks, Inzlicht and Kang2008; Gonzales, Blanton, & Williams, Reference Gonzales, Blanton and Williams2002; Hess, Auman, Colcombe, & Rahhal, Reference Hess, Auman, Colcombe and Rahhal2003; Levy & Langer, Reference Levy and Langer1994; Spencer, Steele, & Quinn, Reference Spencer, Steele and Quinn1999; Steele, Reference Steele1997; Steele & Aronson, Reference Steele and Aronson1995). To illustrate, Spencer et al. (Reference Spencer, Steele and Quinn1999) tested the impact of the stereotype that women are worse at math than men in actual performance. As hypothesized, women performed less well than men in difficult math exercises when the exercises were framed as producing gender differences. In contrast, men and women performed equally well in a condition without stereotype threat.
Stereotype threat has also been studied in people with brain impairment. In a study on 36 participants with a history of mild head injury (Suhr & Gunstad, Reference Suhr and Gunstad2002), the stereotype (i.e., diagnosis) threat group performed worse than the neutral group on general intellect and memory, whereas no differences occurred for basic attention or psychomotor speed. To date, very few studies have looked at stereotype threat in people with mental disorders. In a study on 30 patients with schizophrenia who underwent each a stereotype threat and a control condition, patients’ social skills were rated as worse in the stereotype threat condition by an observer who did not know about the diagnostic status of the patient (Henry, Hippel, & Shapiro, Reference Henry, Hippel and Shapiro2010). To the best of our knowledge, no study has looked at stereotype threat in OCD, yet.
The present study was designed to transfer this line of research to the field of neurocognition in OCD. Neurocognitive assessments are common in OCD research and may be prone to the effects of stereotype threat. This is because, before the start of a study, its rationale must be disclosed to patients, and the rationale may in some cases contain hypotheses about circumscribed brain and neurocognitive impairments in OCD. In view of the mixed findings on neurocognitive performance in OCD and some initial evidence of major influences contributing to secondary impairment such as poor motivation (Moritz et al., Reference Moritz, Klein, Desler, Lill, Gallinat and Schneider2017, Reference Moritz, Hottenrott, Jelinek, Brooks and Scheurich2012) and interference with symptoms (Abramovitch et al., Reference Abramovitch, Dar, Hermesh and Schweiger2012), we aimed to examine whether stereotype threat compromises neuropsychological performance in individuals with OCD.
A hospital environment, where such tests usually are carried out, may induce a strong stereotype threat, thereby potentially creating a ceiling effect. To prevent this, we pursued our hypothesis using an online assessment. Individuals were randomized to either a stereotype threat condition (they were told that neurocognitive deficits exist in mental disorders, for example, OCD) or a control condition (neutral text) and were then administered neuropsychological tests and several self-report scales on self-stigma, subjective cognition, and symptoms. We expected that participants in the stereotype threat condition would display a poorer performance on the neuropsychological tests than participants who were not given such cue. We chose a memory test and a task of inhibitory control as deficits have been implicated in these domains (Chamberlain et al., Reference Chamberlain, Blackwell, Fineberg, Robbins and Sahakian2005; Lipszyc & Schachar, Reference Lipszyc and Schachar2010; Shin et al., Reference Shin, Lee, Kim and Kwon2014).
METHODS
Participants
Participants were drawn from online forums for people with OCD as well as a mailing list of former patients with a verified diagnosis of OCD. The latter subgroup had agreed to be contacted for future research studies. Informed consent was obtained from all participants before the assessment started. The study was approved by the Ethics Committee of the Medical Board Hamburg. The initial sample consisted of 63 participants who had been formerly treated for OCD or had disclosed a formally established diagnosis of OCD (and were still displaying symptoms). All completed the questionnaires and neuropsychological tests. Participants were excluded if they had severe neurological symptoms (e.g., stroke, brain injury), if their age was below 18 or above 70, or if their OCD symptoms were no longer present. Comorbid psychiatric disorders, except for schizophrenia and bipolar disorder, were tolerated. Depression was the most common comorbid disorder.
Data from 13 individuals were discarded from the analyses because of a neurological disorder (n=2; cerebral paresis as a child, stroke), low scores on the Yale-Brown Obsessive Compulsive Scale (Y-BOCS; see below, n=2, score=0), technical problems with the Go/NoGo task (n=3; e.g., the task did not work on an iPad), and age over 70 (n=1). Moreover, data from 5 of these 13 participants were deleted blind to results to balance the subsamples as to age and gender, which were initially significantly different between the stereotype threat and the control condition.Footnote 1 Of the final sample, 24 were allocated to the experimental condition and 26 to the control condition. According to G*Power (Erdfelder, Faul, & Buchner, Reference Erdfelder, Faul and Buchner1996), this sample size is sufficient to yield a significant group difference at a medium effect size (d=.5) with a power of 0.80. No group differences emerged for baseline demographic characteristics and psychopathology (see Table 1). Forty-three participants in the final sample provided information on their current psychotropic medication (none, n=14; antidepressant medication only, n=26; St. John’s Wort, n=1; antidepressant and antipsychotic medication in combination, n=1; other, n=1).
Table 1 Demographic, psychopathological, and neurocognitive data, using Bayesian and conventional statistics
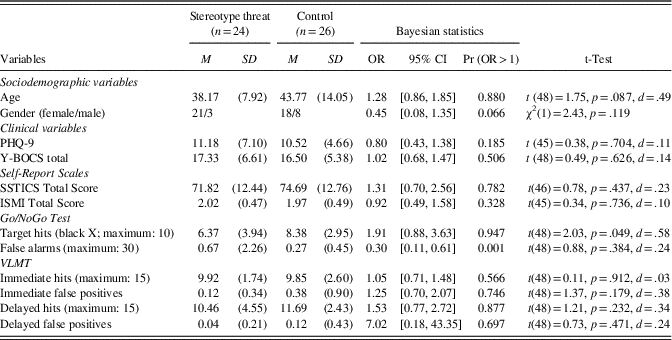
Notes. 95% CI=95% credible interval, Pr (OR>1)=probability that OR is superior to 1; ISMI=Internalized Stigma of Mental Illness (the total score does not include the stigma resistance scale); OR=odds ratio; PHQ-9=Patient Health Questionnaire-9; SSTICS=Subjective Scale to Investigate Cognition in Schizophrenia; VLMT=Verbal Learning and Memory Test; Y-BOCS=Yale Brown Obsessive-Compulsive Scale.
Tasks and Procedure
The online study was set up using the Questback Unipark® software. Individuals received a link to the study and were then randomly assigned to either the experimental group that received a stereotype threat cue or the control group that did not receive a stereotype threat cue. Both groups completed the same battery of neuropsychological tests and questionnaires. The entire assessment lasted approximately 40 min. The only difference between the two conditions was that the experimental group was given the following subtle stereotype threat cue: “Scientific studies have shown that patients with mental disorders (e.g., psychosis, OCD, etc.) have mild brain disorders as well as difficulty concentrating and remembering new information. The two following tests are designed to measure your cognitive abilities”.
In contrast, the control group received the following rather neutral text: “The following two tests in this study are being examined for their usability.” Toward the end of the experimental procedure, participants were asked to state which introductory information (stereotype threat cue, neutral text [control], none) they had been shown. This was done to check whether the cue had been memorized properly or perhaps had been overlooked and whether patients in the control condition had a false memory and remembered a threat cue when in fact none was presented.
Upon completion of the study, participants received a manual that teaches relaxation exercises plus an audio file. This had been announced as an incentive for participation at the beginning of the trial. They were also debriefed about the purpose of the study.
Neuropsychological Tests
Neuropsychological performance was measured by two standard tests that were adapted for online administration. Selective attention and executive functioning were assessed using an online Go/NoGo task, which is frequently regarded as a test of executive functioning and response inhibition (Ritsner, Blumenkrantz, Dubinsky, & Dwolatzky, Reference Ritsner, Blumenkrantz, Dubinsky and Dwolatzky2006; Weisbrod, Kiefer, Marzinzik, & Spitzer, Reference Weisbrod, Kiefer, Marzinzik and Spitzer2000), and deficits in these two domains have been implicated in OCD for many years (Chamberlain et al., Reference Chamberlain, Blackwell, Fineberg, Robbins and Sahakian2005). Participants were asked to respond to a target (“Go”, in this case the black letter “X”) by directly clicking on it and to inhibit responses to distractor (“NoGo”) stimuli (i.e., a red “X” or “+” or a black “+”). Individuals were presented with 10 targets and 30 distracters (10 of each subtype).
Memory was assessed using the German adaptation of the Auditory Verbal Learning Test (Verbal Learning and Memory Test, VLMT) (Helmstaedter, Lendt, & Lux, Reference Helmstaedter, Lendt and Lux2001; Schmidt, Reference Schmidt1996; Volz-Sidiropoulou, Poll, Forkmann, & Gauggel, Reference Volz-Sidiropoulou, Poll, Forkmann and Gauggel2010). Participants were visually presented 15 words in a row for 3 s each. The list of words was shown three times. Participants had to remember and enter as many items as possible (e.g., hat, bell, coffee) in a designated space on the webpage after each presentation of the sequence. The words were easy to visualize; the VLMT thus taps both verbal and nonverbal memory. After each set of 15 words, participants were asked to enter the words they remembered, in any order. After a section in which self-report questionnaires were administered (see below), a delayed recall test was given with no interim presentation of items. This was followed by a recognition test encompassing both the already studied (i.e., old) and the new words. Participants had to assess whether a word had been previously presented. The test–retest reliability of the German version of the VLMT is satisfactory (r=.68–.87; Helmstaedter et al., Reference Helmstaedter, Lendt and Lux2001).
Self-Report Questionnaires
All questionnaires were presented to both groups after the cognitive tasks to rule out the possibility that the items of the scales induced stigma.
Subjective cognitive dysfunction was tapped with the Subjective Scale to Investigate Cognition in Schizophrenia (SSTICS), a 21-item scale that quantifies different cognitive domains (e.g., memory, attention, language, and praxia) in patients (Stip, Caron, Renaud, Pampoulova, & Lecomte, Reference Stip, Caron, Renaud, Pampoulova and Lecomte2003). The SSTICS was originally constructed to tap deficits frequently observed in schizophrenia but also other disorders. Participants are asked to endorse how often they have noticed cognitive impairments in their daily life in the past two weeks using a 5-point Likert scale (very often, often, sometimes, seldom, never). For the current study, the SSTICS was divided into two parts. As mentioned, items related to stigma were shown after completion of the neuropsychological tests, as these might have acted as an additional stereotype threat cue (e.g., Do you have difficulties remembering the names of your medication?” or “Do you forget to take your medication?”).
Self-stigma was assessed by the Internalized Stigma of Mental Illness (ISMI) scale. The ISMI contains subscales measuring alienation, stereotype endorsement, perceived discrimination, social withdrawal, and stigma resistance on a 4-point Likert scale (i.e., strongly disagree, rather disagree, rather agree, strongly agree) (Boyd, Adler, Otilingam, & Peters, Reference Boyd, Adler, Otilingam and Peters2014; Boyd Ritsher, Otilingam, & Grajales, Reference Boyd Ritsher, Otilingam and Grajales2003). The German version of the ISMI shows high internal consistency (Cronbach’s α=.92) as well as good test–retest reliability (r=.71).
The Patient Health Questionnaire (PHQ-9) (Löwe, Kroenke, Herzog, & Gräfe, Reference Löwe, Kroenke, Herzog and Gräfe2004) taps depressive symptoms on a 4-point scale (not at all, on some days, on the majority of days, nearly every day) with reference to the past 2 weeks. It is regarded as a valid and reliable screening instrument for depression (Cronbach’s α=.88).
Statistical Analyses
We used both conventional and Bayesian analysis methods to compare sociodemographic variables, self-report questionnaires, and cognitive performance. Two statistical methods were adopted as there is a growing debate whether “p-value statistics” is outdated and Bayesian statistics is a superior alternative. This debate has not been resolved (Holtmann, Koch, Lochner, & Eid, Reference Holtmann, Koch, Lochner and Eid2016; Ma et al., Reference Ma, Thabane, Kaczorowski, Chambers, Dolovich, Karwalajtys and Levitt2009), so we used these two statistical methods to ensure that our findings will be meaningful years from now if the scientific community ends up favoring one method over the other. For the Bayesian analyses, we used very lowly informative priors for the univariate analyses (normal distribution N [mean ± standard deviation] for the log-OR=N [0; 102]), which amounts to expecting an odds ratio (OR) equal to 1 with a 95% credible interval (CI) of 0.001 to 1000.
Results are presented for each potential predictor as an OR with a 95% CI, with the probability (Pr) of the OR being above 1 (Pr [OR>1]). A large Pr (OR>1) value (e.g.,>0.95,>0.975, or 0.99) must be interpreted as indicating lower values for the individuals in the stereotype threat condition compared to controls. A small value of Pr (OR>1), for instance,<0.05, 0.025, or 0.01, reflects higher values for the individuals in the stereotype threat condition compared to the controls (the stereotype threat was coded with 1 and the control condition with 2). It is worth noting that the probability Pr (OR>1) can be interpreted as 1 – Pr (OR<1). Thus, probability values near 1 and 0 both indicate a significant effect.
RESULTS
The results in Table 1 show that the two groups did not differ in terms of age, sex ratio, or psychopathological scales. Patients showed mild depression and low OCD symptom severity. Most patients had minimal to mild self-stigma, according to the scoring recommendations of Lysaker, Roe, and Yanos (Reference Lysaker, Roe and Yanos2007). The two groups did not differ on medication status (on vs. off), χ2(1)=0.803; p=.370), or on prevalence of comorbid depression, χ2(1)=0.12, p=.729.
Similarly, the two groups did not differ on SSTICS or ISMI scores (see Table 1). With regard to cognitive performance, individuals in the stereotype threat condition had a probability of 94.7% of obtaining fewer hits than the controls on the Go/NoGo task (Bayesian statistics). Using conventional statistics, the groups showed a significant difference. According to Bayesian statistics, there was a 99.9% probability that patients in the stereotype threat condition would have more false alarms than the controls on the Go/NoGo tests. This finding, however, was not observed using conventional statistics (neither parametric nor non-parametric tests), as the data had a Poisson distribution and were not normally distributed. No group difference was observed for the VLMT test with either procedure.
Sensitivity analyses were performed for cognitive tests and examined the effects of priors, expecting that the ORs of the differences between the participants in the stereotype threat condition and the controls would be 1.3, 1.5, or 2. The estimated ORs remained unchanged (data not shown), suggesting that the estimates of ORs were mostly driven by the data we collected and were not sensitive to priors (i.e., expected results).
We then ran multivariate analyses of the cognitive tests after adjusting for age and gender. The Deviance Index Criteria improved in comparison to the univariate models, showing that these variables were relevant predictors. Results of both the Go/NoGo and VLMT tests remained roughly unchanged, with the exception that the probability that individuals in the stereotype threat condition would have fewer hits than the controls on the Go/NoGo task increased from 94.7% (the result of the univariate model; see above) to 96.0%. Using frequentist statistics, group differences numerically increased for the Go/NoGo paradigm, p=.037, to a medium to strong effect (η2 partial=.90).
Finally, we explored the possibility that stereotype threat may interact with the level of internalized stigma by examining the interaction between group and internalized stigma. Results with Bayesian statistics showed no interaction between group and internalized stigma (OR=0.83, CI [0.147–2.734], Pr (OR>1)=0.266). A two-way ANOVA (using median split of ISMI total score; high vs. low) yielded similar results (p>.2).
A manipulation check showed that nine participants could not remember the original instruction and two participants confused the condition (the participant either reported stereotype threat although the participant was in the control condition or vice versa). The results remained unchanged when this was considered in the analyses.
We correlated objective versus subjective scores to explore patients’ metacognitive awareness of their neurocognitive performance. None of the objective neurocognitive measures in Table 1 correlated with the self-report scales. Of note, the VLMT parameters did not correlate with the corresponding SSTICS memory subscale, and, likewise, the Go/NoGo parameters did not correlate with the SSTICS distractibility subscale. The subjective performance (SSTICS total score) was, however, correlated with the PHQ-9 (r=−.463; p<.001) and stigma (r=−.486; p<.001) but not with the Y-BOCS (│r│<.3; p>.05); a trend emerged only for obsessions (r=−.28; p=.053). Self-stigma was correlated with depression (r=.62; p<.001) as well as obsessions (r=.59; p<.001) and, as a result, also with the Y-BOCS total score (r=.47; p=.001). No correlation, however, emerged for compulsions (r=.07; p=.61).
DISCUSSION
To the best of our knowledge, this is the first study examining whether the induction of stereotype threat compromises neuropsychological performance in individuals with OCD. The article was stimulated by equivocal findings on the magnitude and profile of neuropsychological deficits in OCD (Jelinek, Moritz, Heeren, & Naber, Reference Jelinek, Moritz, Heeren and Naber2006; Moritz et al., Reference Moritz, Kloss, Jacobsen, Kellner, Andresen, Fricke and Hand2005; Moritz, Kloss, von Eckstaedt, & Jelinek, Reference Moritz, Kloss, von Eckstaedt and Jelinek2009). Having said this, we need to acknowledge that the majority of evidence favors the notion of neurocognitive impairment in OCD (see introduction). Yet, researchers have recently called for caution in interpreting these findings (e.g., Kalanthroff, Teichert, et al., Reference Kalanthroff, Teichert, Wheaton, Kimeldorf, Linkovski, Ahmari and Simpson2017), as many group differences are minor and do not amount to clinically meaningful deficits, which should only be assumed when an individual performs at least 1 or 1.5 standard deviations below the population mean (Abramovitch & Schweiger, Reference Abramovitch and Schweiger2015).Footnote 2
Several studies have implicated motivation and interference/distraction by symptoms as sources of secondary malperformance (Abramovitch et al., Reference Abramovitch, Dar, Hermesh and Schweiger2012; Moritz et al., Reference Moritz, Klein, Desler, Lill, Gallinat and Schneider2017, Reference Moritz, Hottenrott, Jelinek, Brooks and Scheurich2012). The idea that stereotype threat may play an additional role was born out of the consideration that patients undergoing neurocognitive or neurophysiological assessment for research are usually given information (e.g., when signing the informed consent) that contains statements and/or hypotheses about putative deficits in the disorder. As shown by a plethora of studies in social psychology and health-related contexts, such negative information may compromise performance by means of motivational, affective, physiological, and cognitive processes (Schmader et al., Reference Schmader, Johns and Forbes2008) that divert cognitive resources and attention needed for optimal performance (this account is compatible with the attentional control theory; Eysenck, Derakshan, Santos, & Calvo, Reference Eysenck, Derakshan, Santos and Calvo2007).
As expected, patients in the stereotype threat condition performed worse than those in the control condition on an executive test (Go/NoGo paradigm). Bayesian statistics also found more false alarms in the stereotype threat condition. Unexpectedly, no such differences emerged for verbal memory or any of the other self-report scales. In our view, lack of power may account for these null findings because the sample size did not allow detection of small effects. The null results thus perhaps represent false-negative findings. Moreover, the online study was conducted in participants’ homes or at least in presumably nonclinical environments. This might have reduced the magnitude of stereotype threat when compared to hospital settings where biogenetic research is usually carried out. Stereotype threat may be elicited more reliably in such a “saturated” clinical setting. This argument ties in well with prior studies indicating that a too subtle threat cue may lead to lack of differences (Ozen & Fernandes, Reference Ozen and Fernandes2011).
A related argument is that a large number of participants did not remember the cue. Nevertheless, we decided against a more blatant cue as that might have created a ceiling effect. Yet, the stereotype threat was strong enough to yield effects on inhibitory control. Alternatively, the stereotype threat may have already been deeply internalized at baseline and thus could not be further boosted by the procedure (Corrigan, Watson, & Barr, Reference Corrigan, Watson and Barr2006). We tested this assumption by splitting the group according to self-stigma scores, but this variable did not interact with the condition on any of the measures. Notably, however, this scale does not contain items pertaining to neurocognition.
Another limitation of our study is that the memory test was not ideally chosen as performance deficits are usually stronger with nonverbal than verbal tasks (Abramovitch et al., Reference Abramovitch, Abramowitz and Mittelman2013; Muller & Roberts, Reference Muller and Roberts2005; Tallis, Pratt, & Jamani, Reference Tallis, Pratt and Jamani1999), although a recent meta-analysis reported deficits for verbal memory as well (Shin et al., Reference Shin, Lee, Kim and Kwon2014). Yet, all the memory items were easy to visualize, so the test involves both verbal and nonverbal processing (Moritz et al., Reference Moritz, Kloss, von Eckstaedt and Jelinek2009). The Go/NoGo task was perhaps more sensitive because it is a complex task and competition for cognitive resources and executive functioning plays a central role in the stereotype threat model of Schmader and colleagues (2008). Subsequent research may thus administer neurocognitive tests for which impairment has been shown before (e.g., block design, Rey figure test), and a nonclinical control group (or normative data) is recommended to elucidate whether stereotype threat is the main reason for impairment or instead augments prior deficits.
Future studies might also test participants before and after the induction of the threat cue to rule out the possibility that group differences were already present at baseline. We also must learn more about the mechanisms governing stereotype threat and how long-lived its effect is. Random administration of tasks may prove beneficial in distinguishing whether differences on tasks reflect a putative sensitivity of the task to stereotype threat or to its order in the sequence (the effect may be stronger if it immediately follows the cue). Finally, the two instructions were quite disparate in terms of content and length. Future studies should align the stereotype threat and the control instructions as far as possible to allow more straightforward conclusions.
In line with previous studies in patients with psychiatric disorders (Moritz, Ferahli, & Naber, Reference Moritz, Ferahli and Naber2004), subjective complaints were not correlated with objective deficits, tentatively speaking for metacognitive problems in the OCD sample but were highly correlated with depression and self-stigma. Self-stigma in turn was correlated with depression as well as obsessive thoughts but not with compulsions. This is in accordance with studies suggesting that the taboo content of obsessions is a frequent cause of self-stigma (McCarty et al., Reference McCarty, Guzick, Swan and McNamara2017). Since the Y-BOCS does not differentiate according to content, we could not test the hypothesis that taboo thoughts about aggression, morality, religion, and sexuality have particularly strong connections to self-stigma.
Conclusion
The current study showed that stereotype threat impairs performance on an executive task in individuals with OCD. At the same time, we failed to demonstrate an effect on verbal memory or any of the self-report measures tapping psychopathology, self-stigma, and subjective cognition. Stereotype threat may accordingly represent a confounding variable for some but not all neurocognitive functions. For future studies, it would be worthwhile to test whether null results on memory and self-stigma reflect high baseline internalized stigma or the rather neutral testing environment. Even if subsequent studies confirm a negligible effect of stereotype threat on most domains, caution is warranted in taking neurocognitive test scores as valid proxies for patients’ real cognitive status or even as an index of cognition-related brain impairment, particularly in view of an emerging set of studies highlighting the role of stress, distraction, test anxiety, and motivation in neurocognitive test results (see introduction).
In addition, medications, particularly antipsychotics and tranquilizers (Van Ameringen et al., Reference Van Ameringen, Simpson, Patterson, Dell’Osso, Fineberg, Hollander and Zohar2014), which are often prescribed in OCD, may also exert a detrimental effect on performance. For future research, we recommend not including statements or hypotheses about neuropsychological deficits in informed consent/disclosure forms given to patients as they may elicit stereotype threat.
To conclude, stereotype threat is a new but potentially important field of investigation and may contribute to some of the cognitive deficits displayed by psychiatric patients in neuropsychological tests.



