Introduction
Time-related changes can negatively impact cognitive functioning, affecting an individual’s quality of life and independence (Reas et al., Reference Reas, Laughlin, Bergstrom, Kritz-Silverstein, Richard, Barrett-Connor and McEvoy2019). As a result, current research in medicine and psychology aims at identifying potential preventive measures. Several studies have shown that short-term cognitive training or stimulation can improve cognitive functioning (e.g., Woods et al., Reference Woods, Aguirre, Spector and Orrell2012). The role of physical activity (PA) has also been extensively examined because of its potential to prevent age-related diseases and to maintain or even enhance cognitive functioning.
PA and physical exercise (PE) are defined as movements produced by skeletal muscles resulting in energy expenditure. PE includes planned and structured activities, usually carried out to improve or maintain physical fitness (Caspersen et al., Reference Caspersen, Powell and Christenson1985), while incidental PA is the result of unstructured daily activities, such as work, housekeeping, walking, leisure, etc. (Bherer et al., Reference Bherer, Erickson and Liu-Ambrose2013; Satz, Reference Satz1993).
An active lifestyle and engagement in structured PA certainly keep the body healthy, but they also have an effect on brain, cognition, and mood. A large amount of literature shows how PA can have a positive impact on the brain. For example, it can lead to an increase in gray matter volume (e.g., Arenaza-Urquijo et al., Reference Arenaza-Urquijo, de Flores, Gonneaud, Wirth, Ourry, Callewaert, Landeau, Egret, Mézenge, Desgranges and Chételat2017; Weinstein et al., Reference Weinstein, Voss, Prakash, Chaddock, Szabo, White, Wojcicki, Mailey, McAuley, Kramer and Erickson2012), white matter integrity (e.g., Johnson et al., Reference Johnson, Kim, Clasey, Bailey and Gold2012; Tseng et al., Reference Tseng, Gundapuneedi, Khan, Diaz-Arrastia, Levine, Lu, Huang and Zhang2013), functional brain activity (e.g., Vidoni et al., Reference Vidoni, Gayed, Honea, Savage, Hobbs and Burns2013), and cerebral perfusion (Xu et al., Reference Xu, Jerskey, Cote, Walsh, Hassenstab, Ladino, Clark, Labbe, Gunstad, Poppas, Cohen, Hoge and Sweet2014). PA seems to slow down the process of age-related neuronal and volumetric loss and reduce both lesions in the white matter and myelin loss, promoting better oxygenation and blood supply to the brain (Goenarjo et al., Reference Goenarjo, Bosquet, Berryman, Metier, Perrochon, Fraser and Dupuy2020).
In most studies, PE training resulted in improved cognitive functioning, with the greatest effects observed in executive functions (e.g., Liu-Ambrose et al., Reference Liu-Ambrose, Nagamatsu, Graf, Beattie, Ashe and Handy2010), processing speed and episodic memory (e.g., Audiffren & André, Reference Audiffren and André2019).
The effects of PA on cognition were also investigated in individuals with Mild Cognitive Impairment (MCI, Baker et al., Reference Baker, Frank, Foster-Schubert, Green, Wilkinson, McTiernan, Cholerton, Plymate, Fishel, Watson, Duncan, Mehta and Craft2010; Lautenschlager et al., Reference Lautenschlager, Cox and Kurz2010) and dementia (Angevaren et al., Reference Angevaren, Aufdemkampe, Verhaar, Aleman and Vanhees2008; Barnes, Reference Barnes2015; Blondell et al., Reference Blondell, Hammersley-Mather and Veerman2014; Sofi et al., Reference Sofi, Valecchi, Bacci, Abbate, Gensini, Casini and Macchi2011) demonstrating the effectiveness of PA intervention also in these clinical populations. The primary point is that it seems that improvement is not limited to psychometric tests, but also extends to everyday activities, indicating that PA interventions may be adopted as an alternative treatment for individuals with dementia or MCI.
In order to better understand the huge variability of the behavioral manifestation of cognitive aging or clinical dementia, the concepts of brain reserve, cognitive reserve (CR), and brain maintenance have been introduced (Nyberg et al., Reference Nyberg, Lövdén, Riklund, Lindenberger and Bäckman2012; Satz, Reference Satz1993; Stern, Reference Stern2002). They suggest the existence of a series of processes contrasting cognitive decline. In essence, a greater reserve accumulated over time – during adulthood – would correspond to better cognitive functioning in later life and a greater capacity to cope with age-related detrimental effects (Pettigrew & Soldan, Reference Pettigrew and Soldan2019). The primary sources of CR are education, occupational complexity, and free-time activities. Cognitive stimulating activities contribute to both enhance CR and allow to maintain better cognition. In this study, we are focusing on how physical activities across lifespan enhance motor reserve (MR) capacity and contribute to better cognition.
The MR hypothesis
Most studies have focused on the effects of PA training carried out in specific periods of time. However, some recent findings have shown that such beneficial effects on cognition derive not only from interventions with elderly individuals but also from lifelong exercise carried out regularly since early adulthood. Reas and collaborators (Reference Reas, Laughlin, Bergstrom, Kritz-Silverstein, Richard, Barrett-Connor and McEvoy2019), in a cross-sectional study involving 1826 individuals (60–99 years old), found that regular PE at different times of the lifespan was associated with better late-life functioning in multiple cognitive domains. The strongest association of current PE was observed with executive functions and episodic memory. Furthermore, physically active individuals both in teenage years and in older age performed better than those active only during one of these two periods.
This result led us to think that PA can accumulate over time and represent another kind of reserve, that is, MR, a construct reflecting the PA carried out throughout life. This construct comes from attributing cumulative power to a physically active lifestyle at every stage of life, which may be associated with greater ability to cope with normal or pathological motor skill decline expected in late adulthood (Bastos & Barbosa, Reference Bastos and Barbosa2022; Chung et al., Reference Chung, Lee, Lee and Sohn2020). In line with what has already been repeatedly demonstrated at cognitive level, this construct has been named MR.
As mentioned above and reported in the literature, the factors that contribute to increasing MR might also increase CR. However, it is reasonable to assume that factors that determine high CR (i.e., education, work, cognitively stimulating leisure activities) do not necessarily lead to an increase in MR.
The present study aimed at analyzing the impact on cognitive performance of (a) PA across the lifespan (i.e., MR proxy) and (b) Current Physical Activity (CPA) in healthy adults. We expected that the higher the MR and the CPA, the better the cognitive performance. Moreover, in accordance with the most recent literature, we expected a stronger effect on executive functions.
Methods
The study was conducted in accordance with the Declaration of Helsinki, and it was approved by the Ethical Committee of the School of Psychology of the University of Padua (Protocol n°4109, Numero Univoco = 41D39A3D2D925510CD898DD310574A5A).
Participants
A sample of 75 healthy volunteers were recruited in different organizations that had no connection with any clinical settings. The inclusion criteria considered participants over 50, Italian native speakers without neurological or psychiatric diseases and not in important pharmacological or chemotherapy treatment. Furthermore, their raw score on the Mini-Mental State Examination (MMSE, Folstein, et al., Reference Folstein, Folstein and McHugh1975; Italian version, Magni et al., Reference Magni, Binetti, Bianchetti, Rozzini and Trabucchi1996) had to be greater than or equal to 25 (out of 30) to exclude individuals with potential impairment.
Participants’ education was classified into five levels according to years of schooling. This sub-division was due to the automatic registration of the computerized test battery used in this study (Cognitive Function Dementia – Jahn & Hessler, Reference Jahn and Hessler2020): 1, less than 8 years (9 participants, 12% of the total); 2, from 8 to 10 years (27 participants, 36% of the total); 3, from 10 to 12 years (19 participants, 25.3% of the total); 4, from 12 to 13 years (10 participants, 13.3% of the total); and 5 for more than 13 years (10 participants, 13.3% of the total). See Table 1 for details about participants’ characteristics.
Table 1. The table shows the descriptive statistics of the main characteristics of the two groups of the sample
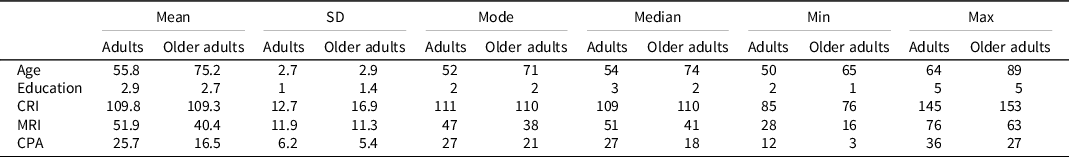
Note. Adults sample size = 37 (22 female, 13 male); Older adults sample size = 38 (23 female, 15 male). SD = Standard Deviation; CRI = Cognitive Reserve Index; MRI = Motor Reserve Index; CPA = Current Physical Activity.
Materials
The materials used to carry out this investigation included four different tools to evaluate participants’ CR, PA, and cognition.
a. The Motor Reserve Index questionnaire (MRIq)
The MRIq is a semi-structured questionnaire administered by a professional, specifically developed for this study to quantify both incidental and structured PA carried out across the lifespan. The MRIq comprises 17 items covering 6 areas (or sections in the questionnaire) and assigns a score to each activity based on frequency and years of practice, starting from 18 years old. The score of each item is calculated in hundredths proportionally to the maximum possible raw scores. For example, a 58-year-old person who has done soft housework activities from the age of 28, will have 30 years of activity (58 minus 28) out of a possible of 40 (58 minus 18). The final score in cents will be 75 (i.e., 30/40 then multiplied by 100). Obviously, a person who carried out daily soft housework activities from the age of 18 will obtain 100 at the item investigating “soft housework activities,” conversely, if the person has never done domestic activities will get 0. The overall score (range: 0–100) is the average of the mean score of each single section. The different sections are described below.
Section I – Housework activities
Three items according to type of housework: soft, for example, sweeping and washing dishes; moderate, for example, ironing, washing floors, or washing clothes by hand; and strenuous, for example, cleaning windows.
Section II – Walking
Three items investigating how much a person walks every day. The participant is asked how many times per week they walk short distances (less than 1 km), long distances (more than 1 km), and how many times they use the stairs.
Section III – Leisure activities
Two items evaluate calorie expenditure when carrying out hobbies.
Section IV – PE
Two items evaluate structured sports activities.
Section V – Care activity
Two items evaluate daily activities aimed at caring for other people or for pets, which involve energy expenditure.
Section VI – Workplace activities
Five items quantify a person’s physical effort at work.
The questionnaire is easy to understand and it took about 5 minutes to be administered.
b. The Current Physical Activity questionnaire (CPAq)
The CPAq was developed using the same items of the MRIq but asking participants to consider the previous twelve months. CPAq as MRIq is administered by an examiner as a semi-structured interview. It comprises 17 items covering 6 areas, 5 of which for all participants and one for those who were still working at the time of the study. For each item, four alternatives based on weekly frequency can be selected: never, rarely (once a week), sometimes (two/three times a week), and often (more than three times a week). The questionnaire is easy to understand and quick to administer (about 5 minutes). The global CPAq score is obtained by summing up the single item scores (range 0–51).
Since MRIq and CPAq were developed ad hoc for this study, we provide some psychometric properties and preliminarily evidence of usability in the Supplementary Materials 1.
c. Cognitive Reserve Index questionnaire (CRIq; Nucci et al., Reference Nucci, Mapelli and Mondini2012, freely available at https://www.cognitivereserveindex.org/)
CRIq is a semi-structured interview measuring the amount of CR acquired during a person’s lifetime. In a single index, the CRI conveys three primary sources of CR: education, working activity, and leisure time activities. The CRI assigns a score to each activity based on the frequency and the number of years of practice. Thus, CRI represents a composite index of CR following the most recent initiatives (e.g., the 2019 Copenhagen Summit on CR). The questionnaire was administered to each participant in its digital form, and it lasted about 10 minutes.
d. Cognitive Function Dementia (CFD; Jahn & Hessler, Reference Jahn and Hessler2020)
The CFD is a comprehensive test battery to evaluate different cognitive functions which provides an index of general cognitive functioning (i.e., Global Cognitive Functioning index). The tests are administered in a standard sequence on a touchscreen computer, and the whole administration lasts about 60 minutes. This battery detects subtle differences among healthy participants, avoiding the ceiling effect. The tests included in the battery are listed and described in the Supplementary Materials – 2.
Procedure
All participants were informed about the general aims of the study and time of administration (about 1 hour and 30 minutes). The MMSE, CRIq, and CFD were administered first and subsequently the participants underwent CPAq and MRIq. All participants signed an informed consent after being explained the experimental procedure.
Data analyses and results
Jamovi version 1.6 software (The Jamovi Project, 2021) and R (version 4.1.0, RStudio Team, 2020) were used for the analyses.
The sample (N = 75), aged between 50 and 89 years old (M = 65.6; SD = 11.7), showed a percentage of females of 60%. The participants’ cognitive reserve index (CRI) ranged from 76 to 153 (M = 110, SD = 16.4); MRI (M = 45.9, SD = 12.9, range = 16–76) and CPA (M = 21.1, SD = 7.40, range = 3–36) were distributed as a Gaussian curve (MRI: S-W p = .69; CPA: S-W p = .67). Age was not correlated with CRI (r = −.07), but it was negatively correlated with MRI (r = −.48) and CPA (r = −.71). CRI did not show any significant correlation with MRI (r = .03) and CPA (r = .04). MRI was positively correlated with CPA (r = .53).
A multiple linear regression approach was adopted. The dependent variable was the Global Cognitive Functioning index, while Age, CRI and MRI or CPA were the predictors. In both models, all predictors were significant within the model (See Table 2 for more details).
Table 2. Values of the model coefficients (standardized β and p-value) and the model fit measures of the four models
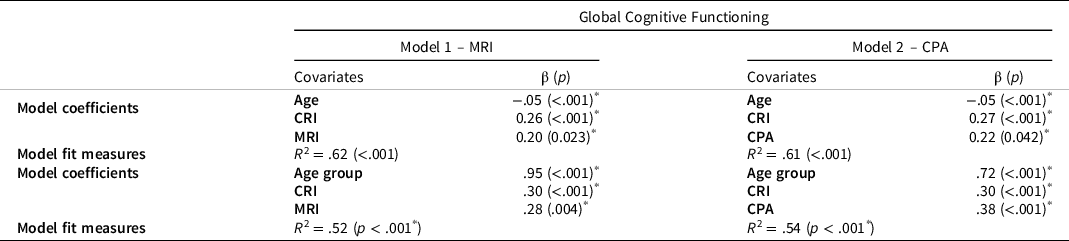
Note. CRI = Cognitive Reserve Index; MRI = Motor Reserve Index; CPA = Current Physical Activity.
* indicates significant F-tests.
With the aim of evaluating the effect of PA in relation to different age groups, the sample was divided into two groups according to their age: Adults (range 50–64 years old, N = 37) and Older adults (range 65–89 years old, N = 38). This division is based on data in the literature (e.g., Salthouse, Reference Salthouse2016) which showed relevant cognitive changes in people when they get to their 60–65s. Two additional regression models were used for the Global Cognitive Functioning index: Model 1, with Age as factor, and CRI and MRI as covariates; and Model 2, with Age as factor, and CRI and CPA as covariates.
Model 1 predicted about 52% of the Global cognitive functioning index variability (R 2 = .52) and all three predictors were significant within the model (β Age = .95; Adults performed better than Older adults; β CRI = .30, p < .001; β MRI = .28, p = .004). MRI was significant also when considered as a single predictor in the model (R 2 = .25, p < .001). The section of the MRIq with the greatest influence on the Global cognitive functioning index was the one concerning workplace activities (R 2 = .22; p < .001).
Model 2 explained about 54% (R 2 = .54, p < .001) of Global cognitive functioning index variability, and all the three predictors were significant within the model (β Age = .72, p < .001; that is, Adults performed better than Older adults; β CRI = .30, p < .001; β CPA = .38, p < .001). CPA was also significant when considered as a single predictor in the model (R 2 = .38; p < .001). The section of CPAq with the greatest influence on the Global cognitive functioning index was the one concerning workplace activities (R 2 = .35; p < .001).
See Table 2 and Figure 1 for more details about the models.
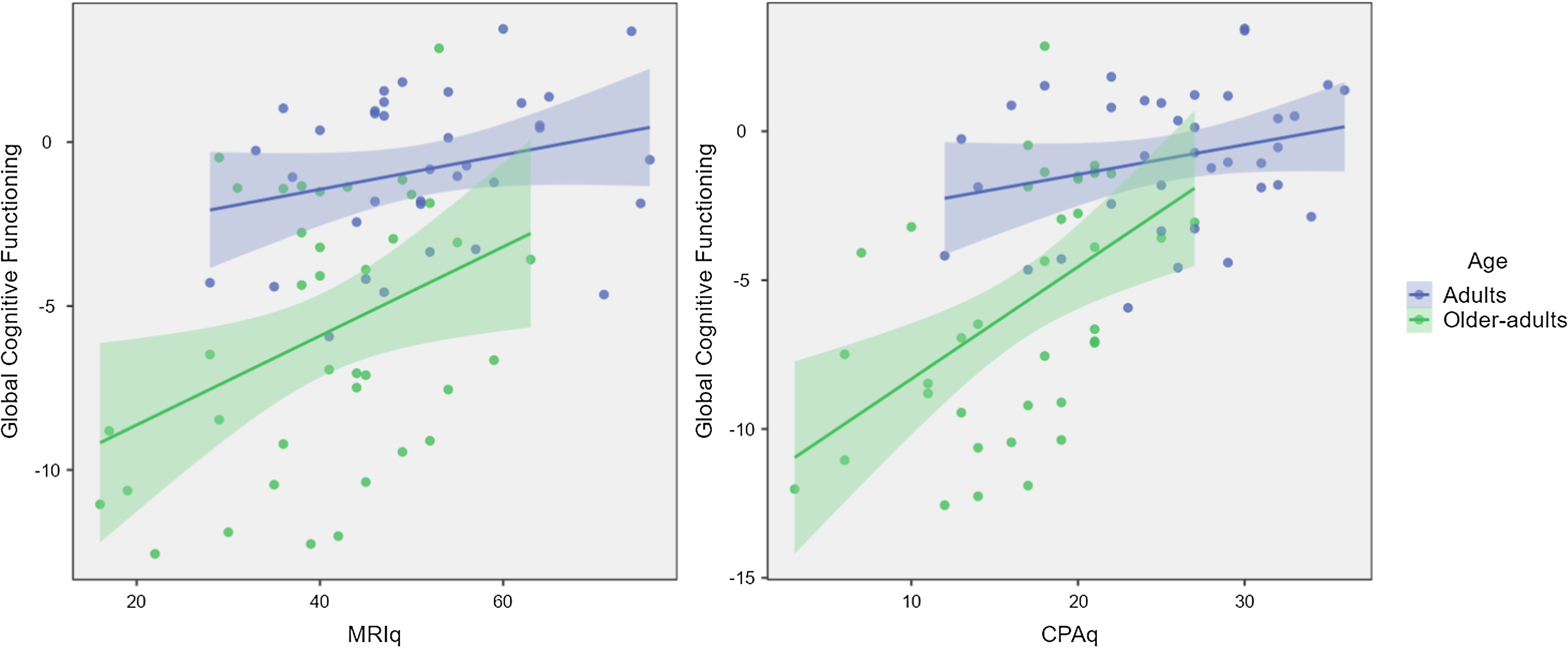
Figure 1. The two graphs show the effect of MRI (on the left-hand-side) and CPA (on the right-hand-side) on Global Cognitive Functioning in the two Age groups.
Single tasks of global cognitive functioning
The effect of MR (Model 1) and CPA (Model 2), including Age and CRI as predictors for each model, was evaluated also on every single task of the CFD battery (complete results are reported in Table 3). The p-values were adjusted for False Discovery Rate (Benjamini & Hochberg, Reference Benjamini and Hochberg1995).
Table 3. The table shows the effect of the predictors in Model 1 (MRI) and Model 2 (CPA) in the 14 different tasks. p-values are adjusted for false discovery rate (Benjamini & Hochberg, Reference Benjamini and Hochberg1995)
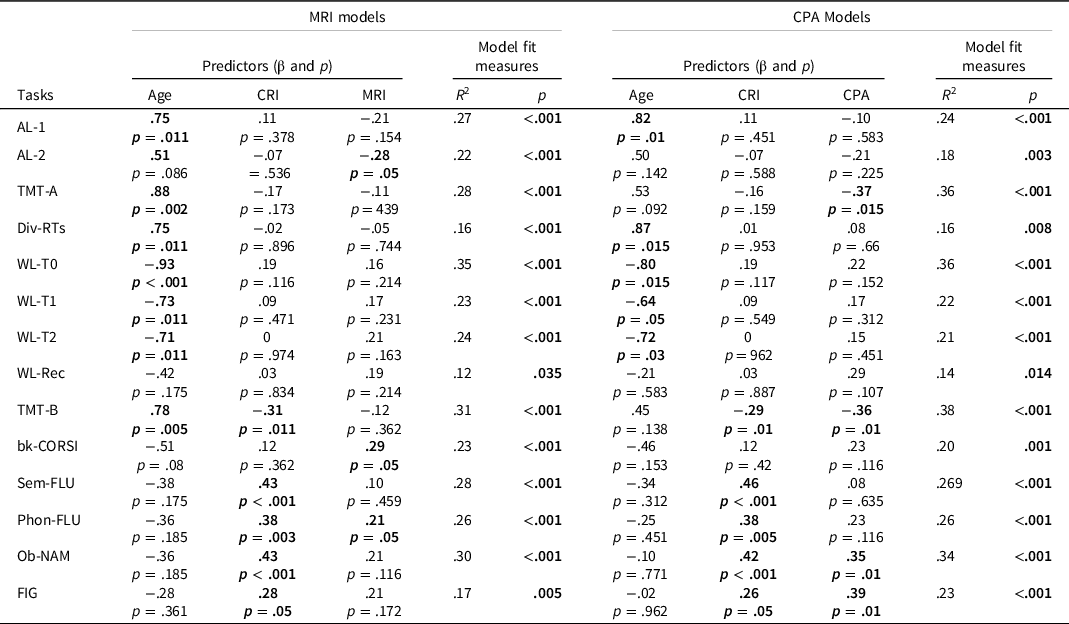
Note. CRI = Cognitive Reserve Index; MRI = Motor Reserve Index; CPA = Current Physical Activity; AL-1 = Simple Alertness; AL-2 = Complex Alertness; TMT-A = Trail-making Test Part A; Div-RTs = Divided Attention task; WL-T0 = Learning Phase of the Auditory Word List Learning task; WL-T1 = Short Term Recall of the Auditory Word List Learning task; WL-T2 = Long Term Recall of the Auditory Word List Learning task; WL-Rec = Recognition Phase of the Auditory Word List Learning task; TMT-B = Trail Making Test part B; bk-CORSI = Backwards Corsi Block-tapping test; Sem-FLU = Semantic Fluency task; Phon-FLU = Phonemic Fluency task; Ob-NAM = Vienna Object Naming task; FIG = Visuoconstruction task.
In Model 1, MRI was significant in predicting Alertness task, backwards CORSI task, and Phonemic Fluency task. In Model 2, CPA was found significant in predicting Trail Making Test-A and Test-B, Object Naming task and Visuoconstruction task (See Table 3 for more details). For each single test, the section concerning the workplace activity of MRI and CPA showed the greatest influence on the dependent variables.
Discussion
This cross-sectional study aimed at verifying whether PA carried out during the lifespan (proxy of MR) and CPA (carried out in the previous 12 months) could predict cognitive performance similarly to CR in adults and older adults (Petkus et al., Reference Petkus, Resnick, Rapp, Espeland, Gatz, Widaman, Wang, Younan, Casanova, Chui, Barnard, Gaussoin, Goveas, Hayden, Henderson, Sachs, Saldana, Shadyab, Shumaker and Chen2019). The expectations were that both MR and CPA would have a critical role in cognition, in addition to age and CR.
Indeed, results show that age affects the Global cognitive functioning index, that is, the Adult group performed better than the Older adult one. The same trend was found for most tasks (i.e., Alertness task, TMT-A and TMT-B, Divided Attention task and all Memory tasks). This result is in line with the literature showing relevant cognitive changes with aging (e.g., Murman, Reference Murman2015).
CR is known to influence cognitive functioning (e.g., Pettigrew & Soldan, Reference Pettigrew and Soldan2019) and in the present study, indeed, high levels of CR predicted better performance overall (i.e., Global cognitive functioning index) and also several tasks in both age groups: TMT-B, Phonemic and Semantic fluency, Object Naming task, and Visuo-constructive task. TMT-B, Semantic and Phonemic fluency tasks have been repeatedly shown to be strictly dependent on CR (e.g., Llinàs-Reglà et al., Reference Llinàs-Reglà, Vilalta-Franch, López-Pousa, Calvó-Perxas, Torrents Rodas and Garre-Olmo2017; Santos Nogueira et al., Reference Santos Nogueira, Azevedo Reis and Vieira2016). The Naming task involves low-frequency and culture-based items (e.g., pretzel) in which CR can play a crucial role (see also Montemurro et al., Reference Montemurro, Mondini, Crovace and Jarema2019). Finally, CR was predictive for the Visuo-constructive task, again because it is highly demanding for individuals without familiarity with the touchscreen as many were in our sample (see Darby et al., Reference Darby, Brickhouse, Wolk and Dickerson2017; Stern, Reference Stern2009; Valenzuela, Reference Valenzuela2019).
For our main research question, data showed that MR predicted: (1) Global cognitive functioning index and performance in some individual tasks; (2) Reaction times to an alert cue (AL-2 task); (3) Backwards CORSI task; and (4) Phonemic fluency task. The MR effect on Global cognitive functioning underscores that PA carried out across the lifespan is crucial in maintaining good-quality cognition, mainly in individuals aged 65 and over. In fact, the MR effect is markedly higher in Older adults compared to Adults. Regarding the specific tasks, in Reaction times with an alert cue (AL-2 task), participants had to inhibit the response to auditory stimulus and correctly respond as quickly as possible to the visual target. This could be considered an inhibition task involving executive functions, and, as the literature reports (e.g., Liu-Ambrose et al., Reference Liu-Ambrose, Nagamatsu, Graf, Beattie, Ashe and Handy2010), the effect of PA is prominent in that domain. This could also explain the results of the bk-CORSI task and the Phonemic fluency task, which are both measures of executive functions, working memory, and cognitive flexibility, respectively.
We found a significant relationship between age and MR as it is plausible to think that, with aging, people tend to reduce the amount of daily PA. However, MR added explained variance within the regression models, meaning that it should be a factor to consider when a cognitive performance needs to be interpreted.
It must be underlined that when MR is taken as a single predictor within the regression models, it predicts the performance in almost all cognitive tasks. Thus, MR can be considered a very important factor in predicting and interpreting cognitive performance in addition to age and CR. Moreover, we found that the MRIq section devoted to Workplace activity is always the most relevant component of MR in predicting the outcome in all tasks, as people usually spend a lot of the time in the workplace in their productive years.
However, a higher MR level does not correspond to a higher CRl level as they are two different and independent types of reserve, both of which contribute in a different way to good cognitive functioning.
Regarding CPA (performed in the previous 12 months), data show that it predicts Global cognitive functioning and performance in several tasks. The effect of CPA on global cognitive functioning underlines that having an active lifestyle in the previous 12 months is important to maintain better global cognition. This finding is in line with the literature that shows that short-term PA training could lead to cognitive improvement. Our findings confirm a previous meta-analysis from Northey et al. (Reference Northey, Cherbuin, Pumpa, Smee and Rattray2018) showing that PE training improved cognitive functioning in participants over 50s, regardless of their cognitive status at baseline.
We found MR and CR two independent predictors of better cognition. On one hand, CR increases brain network connectivity and cognitive functionality (Varela-López et al., Reference Varela-López, Cruz-Gómez, Lojo-Seoane, Díaz, Pereiro, Zurrón, Lindin and Galdo-Álvarez2022); on the other, MR may lead to better brain oxygenation which indirectly sustains better cognition (e.g., Chung et al., Reference Chung, Kwon, Lee, Tack, Lee, Yi and Lee2007, Reference Chung, Lee, Choi, Tack, Lee, Yi, Kim and Lee2008; Kane et al., Reference Kane, Conway, Miura and Colflesh2007). In addition, during aging, individuals are more exposed to cerebrovascular diseases accompanied by several cognitive deficits (e.g., Gorelick et al., Reference Gorelick, Scuteri, Black, Decarli, Greenberg, Iadecola, Launer, Laurent, Lopez, Nyenhuis, Petersen, Schneider, Tzourio, Arnett, Bennett, Chui, Higashida, Lindquist and Nilsson2011). MR might help in preventing age-related motor disabilities, as well as neurodegenerative and vascular diseases, thus promoting better general cognitive functioning and quality of life (see also Siciliano et al., Reference Siciliano, Olivito, Urbini, Silveri and Leggio2022).
Moreover, any type of PA implies the connections of individuals with the environment and their own body. These activities involve numerous and coordinated actions, all requiring cognitive functioning. For example, perception and cognitive estimation of distance, size, shape and weight of objects or spaces; planning of movements of harms and legs and the correct sequence and timing of moves and gauging the necessary strength to reach the goal. Finally, the correct execution of actions itself requires cognitive functioning in modulating the motor response and calibrating the action within the surrounding space.
Thus, motor functions are highly interconnected with sensory and cognitive functions, which mutually co-work in yielding functional behavior.
This work is not exempt from limitations: (1) we used self-reported measures to quantify MR and current status of PA, which may be biased by participants’ own answers; (2) the small size of our sample does not allow to generalize our results, however, it could be a starting point for future investigations; (3) the cross-sectional nature of the study design does not allow to evaluate the genuine effect of PA over time. (4) In addition, we reported only preliminary evidence of the psychometric properties and the usability of the MR Index questionnaire and the current PA questionnaire. In particular, test-retest reliability was calculated in a very small sample.
In conclusion, we found that, similarly to CR, MR and CPA are reliable predictors of global cognitive functioning and of specific tasks involving executive functioning. Maintaining an active lifestyle in older age in terms of PA is just as essential as regular practice across the lifespan, not only for physical well-being, but also for cognition. This highlights the intrinsic relationship between the two.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S135561772300022X
Acknowledgments
None.
Funding statement
None.
Conflict of interests
None.






