Significant outcomes
-
Patients with Niemann-Pick type C (NPC) disease have elevated rates of adhesion interthalamica (AI), and when present, it is shorter than controls and correlates inversely with duration of illness.
-
There were no differences in size of the cavum septum pellucidum in NPC patients compared to controls.
-
This difference suggests that an early rather than late neurodevelopmental disturbance may occur in NPC patients, as may occur in schizophrenia, in addition to accelerated brain atrophy.
-
This supports the notion that NPC in adults may have both neurodegenerative and neurodevelopmental components.
Key limitations
-
This study was cross-sectional rather than longitudinal.
-
The small sample size may limit the capacity to detect true change across both structures.
-
Higher resolution imaging may have the power to more sensitively detect change.
Introduction
Niemann-Pick disease type C (NPC) is an autosomal recessive neurometabolic disorder of intracellular cholesterol trafficking caused by a mutation in the NPC1 or NPC2 genes (Carstea et al., Reference Carstea, Morris, Coleman, Loftus, Zhang, Cummings, Gu, Rosenfeld, Pavan, Krizman, Nagle, Polymeropoulos, Sturley, Ioannou, Higgins, Comly, Cooney, Brown, Kaneski, Blanchette-Mackie, Dwyer, Neufeld, Chang, Liscum, Strauss, Ohno, Zeigler, Carmi, Sokol, Markie, O'Neill, van Diggelen, Elleder, Patterson, Brady, Vanier, Pentchev and Tagle1997), with a combined estimated prevalence of approximately 1:89,000 live births (Wassif et al., Reference Wassif, Cross, Iben, Sanchez-Pulido, Cougnoux, Platt, Ory, Ponting, Bailey-Wilson, Biesecker and Porter2016), although more recent studies have suggested that a later-onset phenotype may have a significantly higher incidence of 1/20,000–40,000. The disruption of intracellular cholesterol trafficking results in accumulation of unesterified cholesterol and glycosphingolipids/gangliosides in lysosomes, particularly in the brain, spleen and liver. In children, the disorder presents with neonatal jaundice and splenomegaly, developmental delay, seizures and gelastic cataplexy; in adults, it more commonly presents with ataxia, dystonia, schizophrenia-like psychosis and vertical gaze palsy (Walterfang et al., Reference Walterfang, Fietz, Fahey, Sullivan, Leane, Lubman and Velakoulis2006b; Sévin et al., Reference Sévin, Lesca, Baumann, Millat, Lyon-Caen, Vanier and Sedel2007; Vanier, Reference Vanier2015).
A range of neuropathological changes occur in the brains of individuals with NPC, some of which are common to other lysosomal storage disorders (LSDs), such as meganeurite formation, neuronal distension, axonal swelling and spheroid formation and ectopic dendritogenesis (Walkley & Suzuki, Reference Walkley and Suzuki2004). However, significant overlap occurs with other neurodegenerative disorders, including altered amyloid processing (Arenas et al., Reference Arenas, Garcia-Ruiz and Fernandez-Checa2017), and tauopathy including paired helical filament (PHF)-type neurofibrillary tangles (Suzuki et al., Reference Suzuki, Parker, Pentchev, Katz, Ghetti, D'Agostino and Carstea1995; Love et al., Reference Love, Bridges and Case1995). Brain regions most affected by these pathological changes, and where neuronal loss is greatest, include cerebellum, brainstem, hippocampus, thalamus and basal ganglia most particularly (Walkley et al., Reference Walkley, Baker, Rattazzi, Haskins and Wu1991); these regions are where the greatest ganglioside excess (Zervas et al., Reference Zervas, Dobrenis and Walkley2001; German et al., Reference German, Quintero, Liang, Ng, Punia, Xie and Dietschy2001), tangle formation (other than the cerebellum) (Love et al., Reference Love, Bridges and Case1995; Suzuki et al., Reference Suzuki, Parker, Pentchev, Katz, Ghetti, D'Agostino and Carstea1995) and neuroinflammation (Pressey et al., Reference Pressey, Smith, Wong, Platt and Cooper2012) occurs.
Previous neuroimaging studies in adult NPC patients have largely confirmed these changes, suggesting grey matter volume losses occur in these subcortical regions and progress over time (Walterfang et al., Reference Walterfang, Fahey, Desmond, Wood, Seal, Steward, Adamson, Kokkinos, Fietz and Velakoulis2010; Walterfang et al., Reference Walterfang, Macfarlane, Looi, Abel, Bowman, Fahey, Desmond and Velakoulis2012; Walterfang et al., Reference Walterfang, Patenaude, Abel, Kluenemann, Bowman, Fahey, Desmond, Kelso and Velakoulis2013; Bowman et al., Reference Bowman, Walterfang, Abel, Desmond, Fahey and Velakoulis2015; Masingue et al., Reference Masingue, Adanyeguh, Nadjar, Sedel, Galanaud and Mochel2017), but are also accompanied by widespread white matter changes (Trouard et al., Reference Trouard, Heidenreich, Seeger and Erickson2005; Walterfang et al., Reference Walterfang, Fahey, Desmond, Wood, Seal, Steward, Adamson, Kokkinos, Fietz and Velakoulis2010; Lau et al., Reference Lau, Lee, Miyamoto, Jung, Yanjanin Farhat, Yoshida, Mori, Gropman, Baker and Porter2016; Masingue et al., Reference Masingue, Adanyeguh, Nadjar, Sedel, Galanaud and Mochel2017), particularly affecting midline structures such as the corpus callosum (Trouard et al., Reference Trouard, Heidenreich, Seeger and Erickson2005; Walterfang et al., Reference Walterfang, Fahey, Abel, Fietz, Wood, Bowman, Reutens and Velakoulis2011; Lee et al., Reference Lee, Apkarian, Jung, Yanjanin, Yoshida, Mori, Park, Gropman, Baker and Porter2014; Masingue et al., Reference Masingue, Adanyeguh, Nadjar, Sedel, Galanaud and Mochel2017), and the most significant atrophy of subcortical grey matter structures is seen in the thalami (Walterfang et al., Reference Walterfang, Fahey, Desmond, Wood, Seal, Steward, Adamson, Kokkinos, Fietz and Velakoulis2010; Masingue et al., Reference Masingue, Adanyeguh, Nadjar, Sedel, Galanaud and Mochel2017), which abut the midline. These changes have been well described and are largely consistent across studies; however, what is not known is whether these changes arise as a result of pathological changes affecting normally developed brain structures, or whether there are more subtle neurodevelopmental changes that occur in the brains of some adult NPC patients that may then be affected by degenerative change (Rego et al., Reference Rego, Farrand, Goh, Eratne, Kelso, Mangelsdorf, Velakoulis and Walterfang2019); many adult patients demonstrate subtle developmental changes in childhood prior to the onset of frank cognitive, neuropsychiatric or motor symptoms during adulthood (Walterfang et al., Reference Walterfang, Fietz, Fahey, Sullivan, Leane, Lubman and Velakoulis2006a; Sévin et al., Reference Sévin, Lesca, Baumann, Millat, Lyon-Caen, Vanier and Sedel2007; Vanier, Reference Vanier2010).
One way of indexing possible early neurodevelopmental change is to examine brain structural changes that have shown to be markers of subtle neurodevelopmental anomalies in other adult brain disorders, particularly neuropsychiatric illness such as schizophrenia, bipolar disorder or major depression. Alterations in the midline structures adhesio interthalamica (AI) and cavum septum pellucidum (CSP) have been shown to be over-represented in these major psychiatric illnesses, two- to threefold, and may represent markers of subtly altered early-life neurodevelopmental trajectory (Trzesniak et al., Reference Trzesniak, Kempton, Busatto, De Oliveira, Galvao-De Almeida, Kambeitz, Ferrari, Filho, Chagas, Zuardi, Hallak, Mcguire and Crippa2011a; Trzesniak et al., Reference Trzesniak, Oliveira, Kempton, Galvao-De Almeida, Chagas, Ferrari, Filho, Zuardi, Prado, Busatto, Mcguire, Hallak and Crippa2011b; Landin-Romero et al., Reference Landin-Romero, Amann, Sarro, Guerrero-Pedraza, Vicens, Rodriguez-Cano, Vieta, Salvador, Pomarol-Clotet and Radua2016).
The AI, or massa intermedia, is a flattened grey matter band which connects the medial surfaces of the thalami across the third ventricle and which generally fuses by the 13th week of gestation (Rosales et al., Reference Rosales, Lemay and Yakovley1968). The AI varies in size among individuals, absent in about 15–25% of human brains (Samra & Cooper, Reference Samra and Cooper1968; Carpenter & Sutin, Reference Carpenter and Sutin1983) and has been suggested as a marker of developmental problems during early gestation. A CSP is formed by the incomplete fusion of the septum pellucidi (Rakic & Yakovlev, Reference Rakic and Yakovlev1968), which normally occurs within 5 months of birth, usually due to growth of surrounding structures (Sarwar, Reference Sarwar1989). A CSP of <5 mm is common in a large proportion of healthy individuals and may occur in up to 30% of the adult population (Shunk, Reference Shunk1963). An enlarged CSP (≥6 mm) (Takahashi et al., Reference Takahashi, Suzuki, Hagino, Niu, Zhou, Nakamura, Tanino, Kawasaki, Seto and Kurachi2007) is often associated with developmental anomalies of the corpus callosum (Rakic & Yakovlev, Reference Rakic and Yakovlev1968; Shen et al., Reference Shen, Gelot, Moutard, Jouannic, Sela and Garel2015) and alterations in limbic structures such as hippocampus and thalamus (Dremmen et al., Reference Dremmen, Bouhuis, Blanken, Muetzel, Vernooij, Marroun, Jaddoe, Verhulst, Tiemeier and White2019), suggesting neurodevelopmental changes occurring when midline and limbic structures are developing during gestation (Wright et al., Reference Wright, Takei, Rifkin and Murray1995). Limbic structures have been reported to show, at least cross-sectionally, significant volumetric reductions in adult patients compared to controls (Walterfang et al., Reference Walterfang, Fahey, Desmond, Wood, Seal, Steward, Adamson, Kokkinos, Fietz and Velakoulis2010; Walterfang et al., Reference Walterfang, Patenaude, Abel, Kluenemann, Bowman, Fahey, Desmond, Kelso and Velakoulis2013), but the timing of these changes has not yet been examined.
In this study, we sought to examine these mid-line structures in a cohort of well-defined adult NPC patients utilising magnetic resonance imaging. We predicted that NPC patients would show an increased incidence of a missing AI, have a shorter AI, and have a larger CSP.
Methods
Standard protocol approvals, registrations and patient consent
The study was approved by the Melbourne Health ethics committee (approval: 2012.066), and all participants provided written informed consent.
Participants
A total of 18 participants were included in this study, including 9 individuals diagnosed with Niemann-Pick disease type C (NPC) and 9 healthy comparison subjects. Healthy controls were selected from a previous study (Di Biase et al., Reference di Biase, Zalesky, O'Keefe, Laskaris, Baune, Weickert, Olver, Mcgorry, Amminger, Nelson, Scott, Hickie, Banati, Turkheimer, Yaqub, Everall, Pantelis and Cropley2017) based on one-to-one age and gender matching with NPC patients. Participant characteristics are shown in Table 1.
Table 1. Sample population and main measures
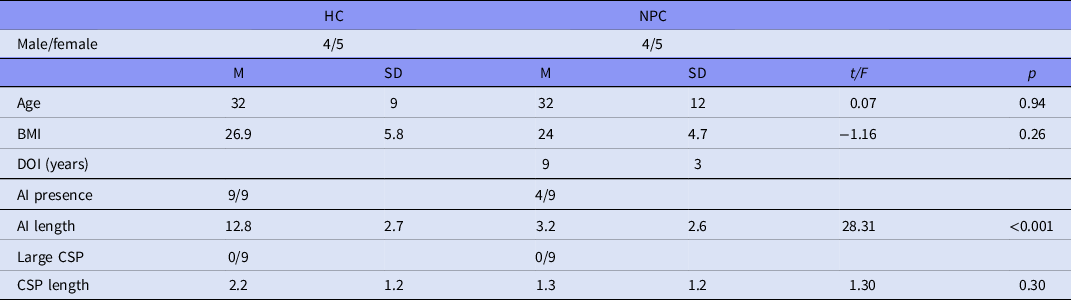
HC, healthy control; NPC, Niemann-Pick disease type C; BMI, body mass index; M, mean; SD, standard deviation; t, t statistic; p, p-value. Equal variances not assumed.
Eligible patients were diagnosed with NPC with initial biochemical testing using filipin staining, with follow-up confirmation via NPC1 genotyping. Exclusion criteria for all participants included a history of head injury, impaired thyroid functioning, diabetes, pregnancy or any contraindication to MRI scanning. Patients’ duration of neurological symptoms was used as a duration of illness (DOI) measure. Additional exclusion criteria for healthy controls, recruited through advertisement, were a history of neurologic or mental illness (personally or in a first degree relative) or alcohol or drug dependence. Healthy controls were interviewed by two trained investigators using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First & Gibbon, Reference First and Gibbon2004) to confirm they had no history of diagnosable psychopathology.
Clinical measures
The following scales were administered within one week of scanning: the SCID (First & Gibbon, Reference First and Gibbon2004) to confirm exclusion criteria for healthy controls and the Iturriaga rating scale (Iturriaga et al., Reference Iturriaga, Pineda, Fernandez-Valero, Vanier and Coll2006) to measure illness severity in NPC patients.
Magnetic Resonance Imaging (MRI) acquisition
T1-weighted and MRI scans were acquired in each participant with a 3-T Siemens Trio at the Murdoch Children’s Research Institute, Royal Children’s Hospital, Parkville Victoria. For T1-weighted images, scanning parameters were as follows: 3D SPGR spoiled gradient T1 weighted, echo time = 3 ms, repetition time = 14 ms, 256 contiguous slices covering whole brain, 1 × 1 × 1 mm voxels. T1-weighted sequences were repeated if images were affected by movement, as determined by the radiologist.
Measurement of structural volumes
To assess the AI and CSP, the images were processed using Dr View software (AJS, Tokyo, Japan) as described previously (Takahashi et al., Reference Takahashi, Suzuki, Hagino, Niu, Zhou, Nakamura, Tanino, Kawasaki, Seto and Kurachi2007; Takahashi et al., Reference Takahashi, Suzuki, Nakamura, Tanino, Zhou, Hagino, Niu, Kawasaki, Seto and Kurachi2008a; Takahashi et al., Reference Takahashi, Suzuki, Tsunoda, Kawamura, Takahashi, Maeno, Kawasaki, Zhou, Hagino, Niu, Tsuneki, Kobayashi, Sasaoka, Seto, Kurachi and Ozaki2008b; Takahashi et al., Reference Takahashi, Suzuki, Zhou, Nakamura, Tanino, Kawasaki, Seal, Seto and Kurachi2008c; Takahashi et al., Reference Takahashi, Yucel, Yung, Wood, Phillips, Berger, Ang, Soulsby, Mcgorry, Suzuki, Velakoulis and Pantelis2008d; Takahashi et al., Reference Takahashi, Yung, Yucel, Wood, Phillips, Harding, Soulsby, Mcgorry, Suzuki, Velakoulis and Pantelis2008e; Takahashi et al., Reference Takahashi, Yucel, Lorenzetti, Nakamura, Whittle, Walterfang, Suzuki, Pantelis and Allen2009). Briefly, brain images were realigned in three dimensions and then reconstructed into entire contiguous coronal images with a 1-mm thickness, perpendicular to the anterior commissure-posterior commissure (AC-PC) line. One rater (TT), who was blind to the subjects' identity and time of scan, counted the number of coronal slices where each midline region was clearly seen. The length of the AI and CSP (in mm) was equal to the number of these slices. We considered the AI as present when it could be identified on three or more slices on both coronal and axial views (Takahashi et al., Reference Takahashi, Suzuki, Nakamura, Tanino, Zhou, Hagino, Niu, Kawasaki, Seto and Kurachi2008a). A CSP equal to or greater than 6 mm was defined as large on the basis of previous analyses (Takahashi et al., Reference Takahashi, Suzuki, Nakamura, Tanino, Zhou, Hagino, Niu, Kawasaki, Seto and Kurachi2008a; Takahashi et al., Reference Takahashi, Suzuki, Zhou, Nakamura, Tanino, Kawasaki, Seal, Seto and Kurachi2008c; Takahashi et al., Reference Takahashi, Yucel, Yung, Wood, Phillips, Berger, Ang, Soulsby, Mcgorry, Suzuki, Velakoulis and Pantelis2008d). Examples of findings from this sample are shown in Fig. 1. Intra- and inter-rater (TT, DS) intraclass correlation coefficients were high for the AI (intra-rater 0.935, inter-rater 0.908) and for the CSP (intra-rater 0.994, inter-rater 9.996).
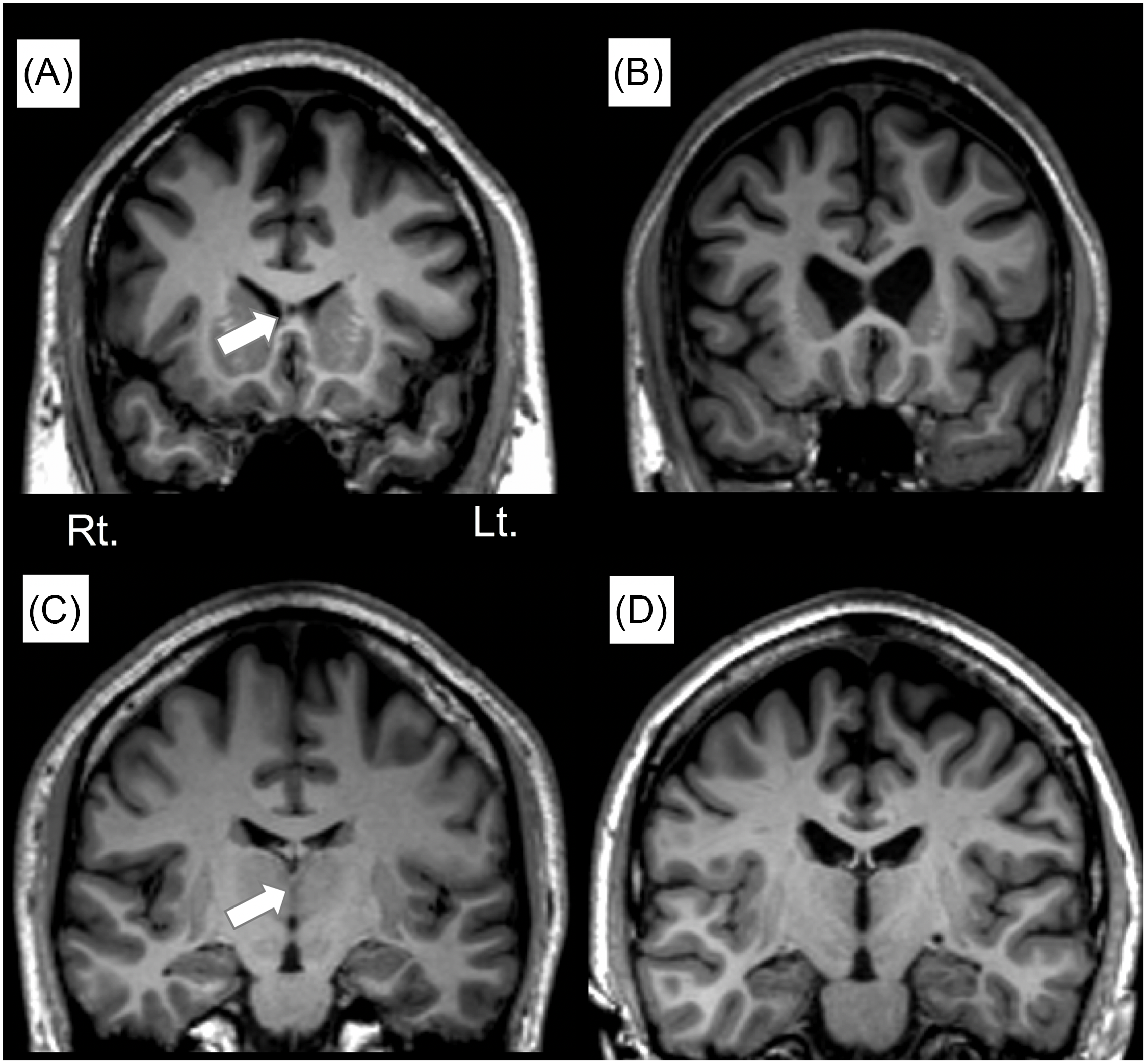
Figure 1. Sample coronal views of the T1-weighted MR images in subjects with (A) and without (B) the cavum septum pellucidum (CSP) and in those with (C) and without (D) the adhesion interthalamica (AI). Arrows indicate the position of each midline brain structure.
Statistical analyses
Between-group differences
Fisher’s exact tests (for cell sizes <5) were used to assess the frequency of the AI and a large CSP. The length of each midline region, which followed a normal distribution (tested by Shapiro–Wilk test), was analyzed using analysis of variance (ANOVA).
Correlations between AI/CSP length and illness measures
The relationships between the midline regions and clinical variables (DOI, illness severity) were examined by Pearson’s partial correlation coefficients controlling for age. DOI and illness severity were normally distributed (Shapiro–Wilk test).
Results
Between-group comparisons
Compared to healthy controls, NPC patients had a missing AI in 5/9 (Table 1, sTable 1), with no control showing a missing AI (Fisher’s exact test, p < 0.05). No patient nor control had a large CSP however. AI length was significantly shorter in the NPC patient group (p < 0.001), but CSP length did not differ between the groups (p = 0.152). These results did not change even when we used non-parametric Mann–Whitney U tests (AI length, U = 0.0, p < 0.001; CSP length, U = 24.5, p = 0.161).
Correlations
Illness severity did not correlate with CSP (p = 0.975) nor AI (p = 0.992) length; duration of illness showed no correlation with CSP length (p = 0.198) but did show a trend to a negative correlation with AI length (p = 0.09).
Discussion
We have demonstrated that adult NPC patients have an elevated rate of an absent AI and a shorter AI compared to matched controls. This is similar to findings seen in first episode and high-risk subjects with schizophrenia, where it has been suggested a shorter or more often absent AI represents an early neurodevelopmental disturbance (Takahashi et al., Reference Takahashi, Suzuki, Zhou, Nakamura, Tanino, Kawasaki, Seal, Seto and Kurachi2008c; Takahashi et al., Reference Takahashi, Yucel, Yung, Wood, Phillips, Berger, Ang, Soulsby, Mcgorry, Suzuki, Velakoulis and Pantelis2008d; Trzesniak et al., Reference Trzesniak, Kempton, Busatto, De Oliveira, Galvao-De Almeida, Kambeitz, Ferrari, Filho, Chagas, Zuardi, Hallak, Mcguire and Crippa2011a). However, we did not identify differences in CSP measures, suggesting that this midline structure may not be a significant marker for any neurodevelopmental disruption in NPC.
Although this was not a longitudinal analysis, the trend towards a correlation between duration of illness and the AI length suggests that this structure may atrophy with time. It has been demonstrated that the AI can show atrophy in both schizophrenia patients and controls (Trzesniak et al., Reference Trzesniak, Schaufelberger, Duran, Santos, Rosa, Mcguire, Murray, Scazufca, Menezes, Hallak, Crippa and Busatto2012; Takahashi et al., Reference Takahashi, Nakamura, Ikeda, Furuichi, Kido, Nakamura, Kawasaki, Noguchi, Seto and Suzuki2013), and it may be a similar process that occurs in adult NPC patients as the AI develops early in gestation but shows some involution with brain ageing (Rosales et al., Reference Rosales, Lemay and Yakovley1968). It is known that the midline nuclei of the thalamus, including the AI, have efferent connections to the amygdaloid nuclei (Graff-Radford, Reference Graff-Radford, Feinberg and Farah1997), and patients with schizophrenia without an AI show smaller amygdaloid volumes (Takahashi et al., Reference Takahashi, Suzuki, Nakamura, Tanino, Zhou, Hagino, Niu, Kawasaki, Seto and Kurachi2008a), suggesting that an absent AI could be a marker of neurodevelopmental disruptions to limbo-thalamic circuitry. It is possible that the trend relationship to illness severity may be a marker of accelerated atrophy in NPC, although longitudinal data are required to confirm this finding.
The lack of findings in the CSP suggest that, if altered early-life neurodevelopment occurs in patients who are diagnosed with NPC in adulthood, it has its impact during the early intra-gestational period when the AI is forming, but not later when the septum pellucidi fuse post-partum. A similar discrepancy between AI and CSP findings – with the former but not latter disrupted – has been shown in major neuropsychiatric disorders such as bipolar disorder and schizophrenia (Takahashi et al., Reference Takahashi, Malhi, Wood, Yucel, Walterfang, Nakamura, Suzuki and Pantelis2010; Takahashi et al., Reference Takahashi, Nakamura, Ikeda, Furuichi, Kido, Nakamura, Kawasaki, Noguchi, Seto and Suzuki2013). However, we recognise that this is a small study, and it may be power that limits our capacity to detect true change across both structures. Additionally, for these small structures, high field strength imaging may be required.
The present study supports the role of AI as a marker of early neurodevelopmental change in individuals who are diagnosed with NPC in adulthood. This fits with suggestions that even adult-onset patients have subtle neurodevelopmental changes that occur early in life, present with subtle developmental signs during childhood (such as learning difficulties or subtle clumsiness (Sévin et al., Reference Sévin, Lesca, Baumann, Millat, Lyon-Caen, Vanier and Sedel2007; Walterfang et al., Reference Walterfang, Fietz, Fahey, Sullivan, Leane, Lubman and Velakoulis2006b)), which may persist due to their non-specificity and thus be overlooked until more obvious motor, psychiatric or cognitive changes occur in adulthood.
Longitudinal studies in larger cohorts, perhaps with pooled scans, that examine patients of different illness severity over time and correlate illness indices with these putative brain markers of altered neurodevelopment and/or neuroprogresssion are warranted.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/neu.2023.43
Author contribution
MW and TT conceived the study. MDB and VC assisted with patient recruitment and data acquisition. TT undertook the neuroimaging analysis. All authors contributed to the manuscript.
Financial support
This study was funded by the award of the Royal Melbourne Hospital Research Medal to Mark Walterfang in 2016, which carried with it a research stipend.
Competing interests
Mark Walterfang has served on advisory boards and received honoraria for consulting from Actelion Pharmaceuticals, Vtesse Pharmaceuticals and Mallinckrodt Pharmaceuticals, who manufacture therapeutic compounds for Niemann-Pick type C.
Tsutomu Takahashi reports no relevant disclosures.
Maria Di Biase reports no relevant disclosures.
Vanessa Cropley reports no relevant disclosures.
Daiki Sasabayashi reports no relevant disclosures.
Michio Suzuki reports no relevant disclosures.
Dennis Velakoulis reports no relevant disclosures.
Christos Pantelis reports no relevant disclosures.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and “the authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.”





