Introduction
Baseline psychopharmacological exposure in young help-seeking subjects at clinical high-risk for psychosis (CHR-P), is a clinically intuitive prognostic modulator with clear implications for individualized risk stratification and more precise outcome prediction (Raballo, Poletti, & Preti, Reference Raballo, Poletti and Preti2020a, Reference Raballo, Poletti and Preti2021). (Please, see ‘Integrative appendix 1’ in the online Supplementary Materials for a synthetic definition of CHR-P and the related notion of transition to psychosis). Recent meta-analytical evidence on CHR-P cohorts revealed that baseline exposure to antipsychotics or antidepressants has a tangible modulating effect on the longitudinal risk of transition to a first-episode of psychosis. Indeed, baseline exposure to antipsychotics is associated with a higher risk of transition to psychosis in comparison to antipsychotic-naïve individuals (29% v. 16%, risk ratio (RR) 1.47) (Raballo, Poletti, & Preti, Reference Raballo, Poletti and Preti2020b), whereas baseline exposure to antidepressant is associated with a lower risk of transition to psychosis in comparison to antidepressant-naïve individuals (13.5% v. 21%, RR 0.71) (Raballo, Poletti, & Preti, Reference Raballo, Poletti and Preti2023).
Given that recent studies indicated a modulating effect of BDZ on outcomes in first-episode psychosis (Arribas, Solmi, Thompson, Oliver, & Fusar-Poli, Reference Arribas, Solmi, Thompson, Oliver and Fusar-Poli2022) as well as in schizophrenia (Strømme et al., Reference Strømme, Mellesdal, Bartz-Johannesen, Kroken, Krogenes, Mehlum and Johnsen2022), the current meta-analytical study was designed to address the specific question whether baseline exposure to BDZ in CHR-P individuals impacts the longitudinal risk of transition to psychosis. (For a succinct overview of the role of anxiety and anti-anxiety treatment in CHR-P, please see ‘Integrative appendix 2’ in online Supplementary materials).
Methods
Study selection
This systematic review and meta-analysis was planned and executed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, & Altman, Reference Moher, Liberati, Tetzlaff and Altman2009; Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow and Moher2021). We searched PubMed/Medline (https://pubmed.ncbi.nlm.nih.gov/) and the Cochrane library (https://www.cochranelibrary.com/) from inception up to 31 December 2022, by using the following key terms: ‘Ultra high risk’ OR ‘Clinical high risk’ and ‘psychosis’ and ‘transition’ OR “conversion. Two authors (MP, AP) evaluated the list of extracted articles and decided about inclusion or exclusion according to the following criteria:
• written in English;
• published in peer-reviewed journals;
• detailing information about samples with people diagnosed at clinical high-risk (CHR) of psychosis based on a validated diagnostic procedure;
• reporting numeric data about the sample and the outcome at a predefined follow-up time; having transition to psychosis as one of the outcomes;
• reporting raw data on BDZ baseline exposure in relation to the transition outcome.
Data extraction
After exclusion of duplicates (including articles repeatedly reporting the results of the same trial or with overlapping samples) and articles that were unrelated to the main topic (i.e. studies on brain imaging or genetic markers), individual studies were included when they matched the inclusion criteria. Discrepancies were resolved consulting a third experienced researcher (AR). The references of the retrieved articles and of the extracted reviews on the topic were scanned to identify potentially missed studies. At the end of this procedure, five independent studies were included in the systematic analysis and the subsequent meta-analysis (Fig. 1: PRISMA Flow chart).
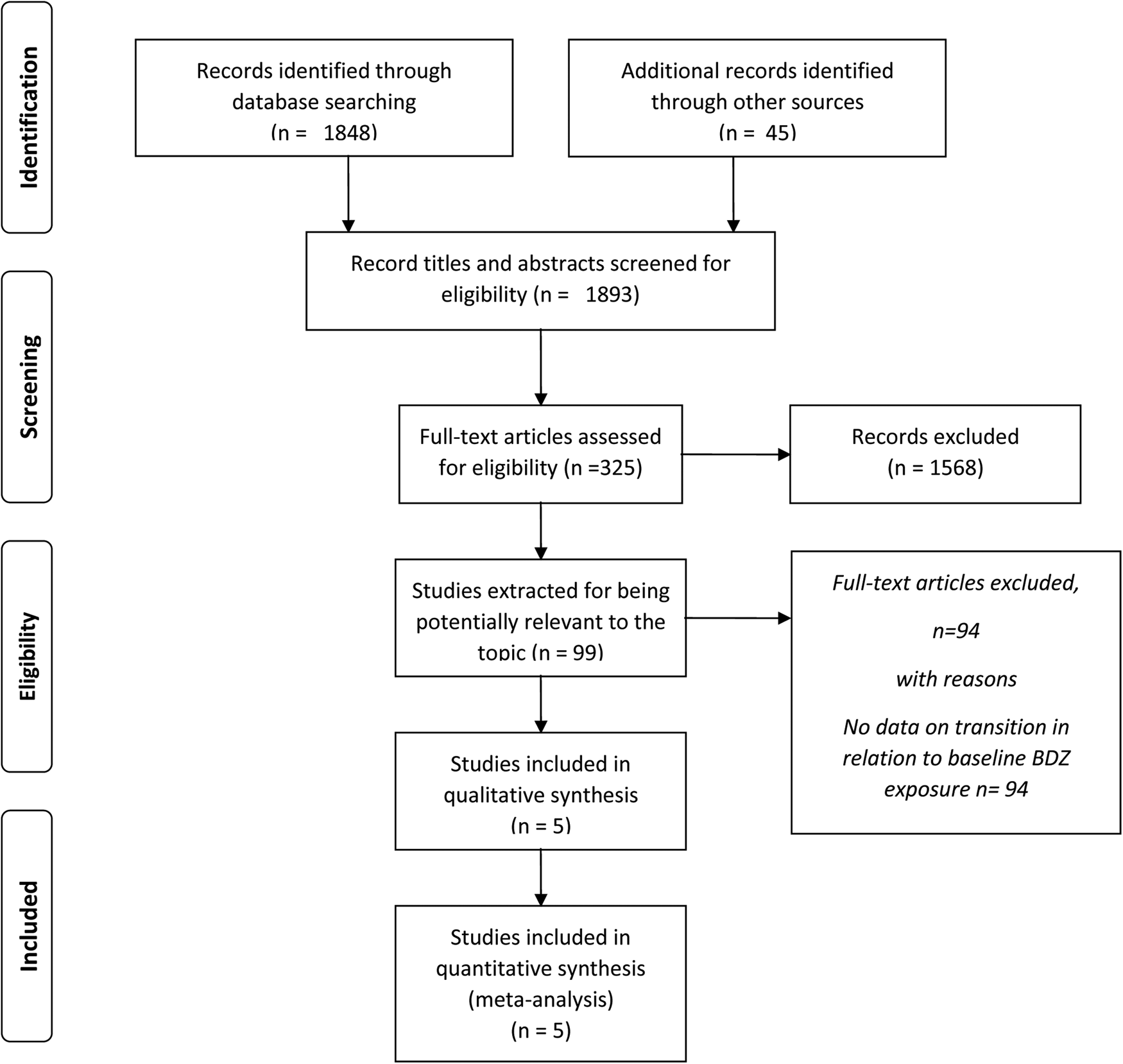
Figure 1. PRISMA 2020 flowchart of studies reporting transition to psychosis in CHR-P help-seeking individuals according to benzodiazepines exposure at baseline.
The following variables were extracted from the included studies: authors and year of publication of the study; location of the study; criteria and instrument for diagnosis; criteria for transition to psychosis; sample size at baseline and at follow-up; mean age in the sample; gender ratio in the sample; data on BDZ exposure (yes/no) on the basis of outcome (transition/no transition); duration of the follow-up; number of cases that transitioned psychosis at the end of follow-up by group; percentage of exposure to AP at baseline.
Quality assessment was rated according to the Newcastle-Ottawa Quality Assessment (Wells et al., Reference Wells, Shea, O'connell, Peterson, Welch, Losos and Tugwell2009). Discrepancies in extraction of data were solved by discussion within the research team. All this procedure was implemented according to an internal protocol.
Data analysis
All analyses were carried out with the ‘meta’ package (Schwarzer, Carpenter, & Rücker, Reference Schwarzer, Carpenter and Rücker2015) and the ‘metafor’ package (Viechtbauer, Reference Viechtbauer2010) running in R version 4.0.2 (R Core Team, 2022).
The outcome of the meta-analysis was the proportion of transition to psychosis. Recent studies showed that one of the most used methods in the meta-analysis of proportions, the variance-stabilizing Freeman and Tukey (Reference Freeman and Tukey1950) double arcsine transformation, might lead to misleading results in a meta-analysis of single proportions due to problems with the back-transformation of the Freeman-Tukey transformation (Schwarzer, Chemaitelly, Abu-Raddad, & Rücker, Reference Schwarzer, Chemaitelly, Abu-Raddad and Rücker2019). Therefore, a random intercept logistic regression model – also known as logit transformation – was used to estimate all single proportions (Stijnen, Hamza, & Ozdemir, Reference Stijnen, Hamza and Ozdemir2010).
Thereafter, we compared the binary outcome of transition to psychosis by group. RR was calculated, and the inverse variance method was used for pooling (Fleiss, Reference Fleiss1993). Between studies variance and variance of the effect size parameters across the population were estimated with the tau-squared statistics using Empirical Bayes estimator (Veroniki et al., Reference Veroniki, Jackson, Viechtbauer, Bender, Bowden, Knapp and Salanti2016); its 95% confidence interval (CI) was calculated by using the Q-Profile method (Viechtbauer, Reference Viechtbauer2010) with Knapp and Hartung (Reference Knapp and Hartung2003) correction. Continuity correction of 0.5 was expected to be applied in studies with zero cell frequencies.
Both common (aka fixed-) and random-effects summary estimates were reported, along with a corresponding 95% CI for each outcome in forest plots. In the interpretation of the results, we gave preference to the fixed-effects model. Since all studies had an observational design, our main goal was to make a conditional inference only about the studies included in the meta-analysis (Viechtbauer, Reference Viechtbauer2010), and the estimates that can be drawn from a common effects model provide perfectly valid inferences under heterogeneity when the inference is limited to the investigated studies (Hedges & Vevea, Reference Hedges and Vevea1998). Moreover, the common effects model does not inflate the role of small studies as the random-effects model does (Borenstein, Hedges, Higgins, & Rothstein, Reference Borenstein, Hedges, Higgins and Rothstein2010). Finally, in modeling heterogeneity in the studies, the random-effects model loses power compared to the common effects model (Jackson & Turner, Reference Jackson and Turner2017).
In all analyses, heterogeneity was assessed with Cochran's Q and I 2 statistics (Huedo-Medina, Sánchez-Meca, Marín-Martínez, & Botella, Reference Huedo-Medina, Sánchez-Meca, Marín-Martínez and Botella2006). A low p value (i.e. p < 0.10) of the Q statistic indicates that variation in the study-specific effect estimates is due to heterogeneity beyond that depending on sampling error (Borenstein, Reference Borenstein2020). The I 2 statistic measures the extent to which the variance in observed effects reflects variance in true effects rather than sampling error (Viechtbauer, Reference Viechtbauer2010). The higher the I 2, the greater the impact of the variance in true effects. According to an agreed rule-of-thumb, I 2 values 0 to 40% might not be important; 30 to 60% may represent moderate heterogeneity; 50 to 90% may represent substantial heterogeneity (Ryan, Reference Ryan2016).
To control the adequacy of the models and outlier detection, we used the radial plot (Galbraith, Reference Galbraith1988). The plot shows the inverse of the standard errors on the horizontal axis against the observed effect sizes or outcomes standardized by their corresponding standard errors on the vertical axis. Studies with estimates that were beyond two standard deviations from the common estimates were assumed to have a poor fit with the model.
The funnel plot was used to graphically assess publication bias. Since there were less than 10 studies, the Egger's test (Egger, Davey Smith, Schneider, & Minder, Reference Egger, Davey Smith, Schneider and Minder1997), and the Begg's test (Begg & Mazumdar, Reference Begg and Mazumdar1994) were not used to statistically test publication bias. For the same reason, we did not use meta-regression techniques to evaluate the impact of the clinical variables that were indicated by our protocol: gender ratio, mean age of the sample, overall sample size, duration of follow-up, concomitant percentage of exposure to AP, AD, MS, and BDZ at baseline, and the quality of the study. We also planned sensitivity analyses with respect to baseline levels of depression, baseline levels of psychotic symptoms, co-morbidity for depressive and/or for anxiety disorders. However, this information was rarely reported in the studies preventing us from running these analyses.
Results
Search results
The literature searching process and study identification are summarized in Fig. 1. Briefly, the initial search identified 1893 records, and study selection procedures yielded five articles (Table 1) reporting on meta-analyzable information as regards baseline BDZ exposure in relation to the binary outcome at follow up (transition/no transition).
Table 1. Studies included in the meta-analysis and reporting raw baseline data on BDZ exposure in relation to transition to psychosis

Legend: AD, Antidepressants; AP, Antipsychotics; BDZ, Benzodiazepines; CHR-P, Clinical High-Risk for Psychosis; s.d., Standard Deviation.
Overall, studies included participants from United States (one study), and four from Europe (one each from Austria, Denmark, Italy, Netherland). All studies included details about age and gender ratio. Studies do vary hugely as far as sample size and time to follow-up, as well as in terms of age and gender ratio were concerned.
Mean age in the five studies was 19.7 ± 4.3, ranging from 15.3 to 24.3 years old. Proportion of females was 50% on average, ranging from 40% to 68%. There were two studies with a sample including exclusively children or adolescents, and three studies with only adult participants (aged 18 years old and older). Sample size at baseline ranged from 39 to 764, with an average sample size of 210. Sample size at follow up ranged 39 to 431, being on average 134. Time to follow-up was up to 12 months in four studies, 36 months in one study.
As far as the tool for the diagnosis was concerned, there were two studies using the Comprehensive Assessment of At Risk Mental States (CAARMS; Yung et al., Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio and Buckby2005), one study using the Positive and Negative Syndrome Scale (PANSS; Kay, Fiszbein, and Opler, Reference Kay, Fiszbein and Opler1987), and two studies using the Structured Interview for Prodromal Syndromes (SIPS; McGlashan, Reference McGlashan2001). Quality was good in four studies and poor in one study (see Table 1 and online Supplementary Table S1 for details).
The proportion of participants with exposure to benzodiazepines (BDZ) at baseline ranged from 5.5% (one study) to 46.2%, with an average of 16.8%.
In three studies, participants had a concomitant exposure to antipsychotics at baseline (average: 26.3%; range: 20.4 to 33.6%); they were also exposed to antidepressants in all five studies (35.0%; 16.6 to 67.7%); in four studies they were also exposed to mood stabilizers (13.2%; 3.4 to 30.8%). We found no information on whether the same participant was exposed concomitantly to BDZ and antidepressants, BDZ and antipsychotics, or BDZ and mood stabilizers. For a fraction of them, this was likely.
At the end of the period of observation, i.e., the follow-up as reported in the study, 28.4% (95% CI 19.7 to 39.1%) participants developed psychosis among the cases (online Supplementary Fig. S2 in supplementary material) against 9.3% (7.3 to 11.9%) among the controls (online Supplementary Fig. S3 in supplementary material).
Risk ratio estimates of transition to psychosis by exposure to benzodiazepine at baseline
CHR-P participants who were already under BDZ treatment at baseline had more than double chance of transition to psychosis than CHR-P participants who were BDZ-naïve. The RR was 2.42 (95% CI 1.38 to 4.23) in the common effects model (z = 3.09; p = 0.002), and 2.40 (1.53 to 3.77) in the random-effects model (z = 5.40; p = 0.006; tau-squared = 0.0) (Fig. 2).
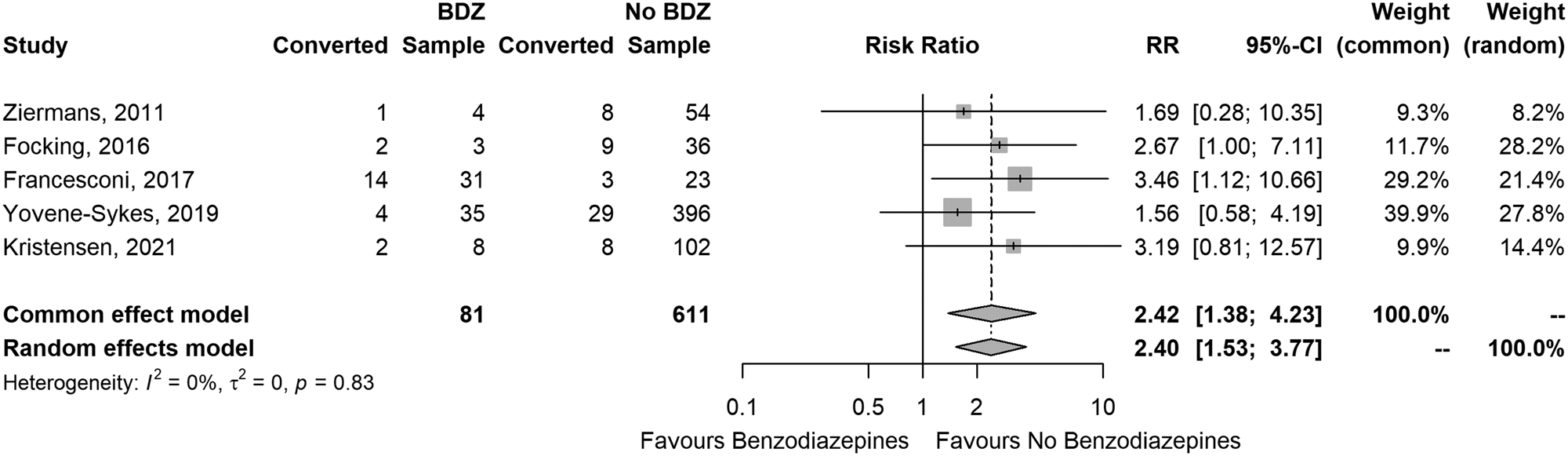
Figure 2. Forest plot of the risk ratio of conversion to psychosis between CHR who were or were not exposed to benzodiazepines at baseline.
There was no relevant heterogeneity: Cochran's Q = 1.49; df = 4; p = 0.828; I 2 = 0.0% (95% CI 0.0–79%). Radial plot indicated good fit of the model (online Supplementary Fig. S4 in the supplementary material), and the funnel plot looked reasonably symmetric (online Supplementary Fig. S5 in supplementary material).
Discussion
The results of the current study indicate that baseline exposure to BDZ in CHR-P individuals has a prognostic modulatory effect on their meta-analytic risk of transition to psychosis at follow-up. In particular, BDZ-exposed CHR-P had more than double transition rate to psychosis compared to non-BDZ exposed CHR-P at baseline. Overall, despite the relatively small group of available studies (which reflects the broader issue of sub-optimal transparency in the field (Raballo et al., Reference Raballo, Poletti and Preti2020a), the converging estimates of both the common and the random-effects models indicate the robustness of the results of this meta-analysis. The model had a good fit, and the funnel plot was reasonably symmetric to suggest there was no relevant publication bias.
Albeit straight inferential causative directions are limited by the observational nature of the studies, some hypotheses can be advanced to explain these meta-analytical findings. BDZ are frequently co-prescribed with antipsychotics in patients with schizophrenia (Längle et al., Reference Längle, Steinert, Weiser, Schepp, Jaeger, Pfiffner and Kilian2012). Often, they are used to treat severe anxiety and agitation or aggression (Sim, Sweetman, Kapur, & Patel, Reference Sim, Sweetman, Kapur and Patel2015). Thus, the use of BDZ in CHR-P might be a clinical proxy of perceived severity from the viewpoint of the treating staff. Similarly, BDZ prescription may indicate more severe symptoms (although still below the clinical threshold for initial CHR-P ascription) and thereby a higher risk of transition at follow-up. In a similar clinical vein, prescription of BDZ in CHR-P might be preferred to antipsychotics because of their better tolerability. However, there is no evidence of efficacy of standalone treatment of BDZ in psychosis (Sim et al., Reference Sim, Sweetman, Kapur and Patel2015), although it has been suggested that they might decrease dopamine acting on GABAA receptors (Möhler, Reference Möhler2006). Nevertheless, exposure to BDZ in patients with schizophrenia was related to a dose-response relationship with premature mortality in longitudinal studies (Tiihonen, Mittendorfer-Rutz, Torniainen, Alexanderson, & Tanskanen, Reference Tiihonen, Mittendorfer-Rutz, Torniainen, Alexanderson and Tanskanen2016). In the long term, BDZ do not possess a favorable prognostic profile in schizophrenia-spectrum psychosis.
It should be noted that in CHR-P individuals, baseline exposure to antipsychotics is associated with a greater risk of transition to psychosis both at a meta-analytical level (Raballo et al., Reference Raballo, Poletti and Preti2020b; Raballo, Poletti, & Preti, Reference Raballo, Poletti and Preti2023) and in subsequent field testing and reanalysis of available cohorts (Preti et al., Reference Preti, Raballo, Meneghelli, Cocchi, Meliante, Barbera and Percudani2022; Zhang et al., Reference Zhang, Raballo, Zeng, Gan, Wu, Wei and Wang2022), and it might be that BDZ – although this info is omitted in the source literature - could have been co-administered with antipsychotics, thereby capturing part of the AP-related pro-transition effect.
Furthermore, BDZ themselves are also related to a high risk of misuse and abuse (Tang & Davies, Reference Tang, Davies, Riederer, Laux, Nagatsu, Le and Riederer2022), it is well-known that withdrawal from BDZ may precipitate positive psychotic symptoms such as hallucinations (Fruensgaard, Reference Fruensgaard1976; Tang & Davies, Reference Tang, Davies, Riederer, Laux, Nagatsu, Le and Riederer2022). The specific testing of this sub-hypothesis (i.e. elicitation of positive symptoms due to BDZ-withdrawal during the follow up) however could not be conducted since the source studies did not specify whether baseline BDZ exposure was maintained or suspended in the follow-up.
A known effect of BDZ, via activation of the GABAA receptor, is the reduction of serotonin turnover (Lim et al., Reference Lim, Sproule, Zahra, Sunderji, Kennedy and Rizvi2020). There is robust evidence in the animal that this action can attenuate the effects of antidepressants in serotonin-mediated models (Lim et al., Reference Lim, Sproule, Zahra, Sunderji, Kennedy and Rizvi2020; Wise, Berger, & Stein, Reference Wise, Berger and Stein1972). There is some meta-analytic evidence of a protective effect of antidepressants on the risk of transition to psychosis in CHR-P help-seekers (Raballo et al., Reference Raballo, Poletti and Preti2023). Thus, the action of BDZ on serotonin, although related to their anti-anxiety effects, could antagonize the favorable prognostic effects of antidepressants in this population.
We lacked the details of the distribution by age and gender of participants according to their baseline exposure to BDZ. Thus we could not do a sensitivity analysis or perform a meta-regression due to the limited sample size, hence lack of power.
It should be noted that the proportion of females in the subgroup of studies included in this meta-analysis was 50%, which was slightly higher than in similar meta-analyses (41% in Raballo et al., Reference Raballo, Poletti and Preti2023; 42% in Raballo et al., Reference Raballo, Poletti and Preti2023). This might depend on women being more prone to treatment acceptance with BDZ (Milani, Raji, Chen, & Kuo, Reference Milani, Raji, Chen and Kuo2021). In the analyzed studies, ages ranged from 15.3 to 24.3 years old. Younger people are less likely to receive a prescription for BDZ than adults (Piovani, Clavenna, & Bonati, Reference Piovani, Clavenna and Bonati2019).
While age seems not to affect the risk of transition to psychosis (Salazar de Pablo et al., Reference Salazar de Pablo, Radua, Pereira, Bonoldi, Arienti, Besana and Fusar-Poli2021), there is some meta-analytic evidence that women have a reduced risk of transition to psychosis from the CHR-P status than men (He et al., Reference He, Wang, Hou, Guo, Huang, Zhao and Li2023). We cannot exclude that disparities in age and gender across samples might influence disease prognosis via treatment acceptance, which might affect the risk of transitioning to psychosis.
More investigation of gender differences in the early stage of psychosis is warranted, both because women remain an underserved minority in early intervention services (Ferrara & Srihari, Reference Ferrara and Srihari2021), and because of their known sensitivity to drugs' adverse effects and additional specificity in care issues, such as domestic abuse, and reproductive and parenting issues (Seeman, Reference Seeman2021).
Strengths and limitations
This meta-analysis adopts of state-of-the-art statistics and procedures and, since the included studies were not primarily aimed to address the issue of the effect of BDZ for transition to psychosis, the results are partially free from a confirmation bias. However, the naturalistic feature of these studies is also a limitation of the meta-analysis, since the prescription of BDZs was at the discretion of the treating staff, and the confounding factors that affect observational studies cannot be ruled out as it happens in the randomized-controlled trials. Although largely secondary to the reporting lacunae of the source literature, several additional limitations must be considered in interpreting the current results. We were unable to analyze the impact of other non-BDZ drugs, since the studies – due to an unfortunate yet widespread habit in the field - did not report more granular information (e.g. on the type and dose of medications as well as whether different medications were or not concomitantly prescribed). Moreover, in the studies, there was scant or no information on whether the prescribed BDZs at baseline were maintained up to the follow-up. We also lacked details about the profile of symptoms at baseline by drug prescription, thus we were unable to determine whether BDZs were effectively prescribed to CHR-P help-seekers with more severe symptoms, e.g., with higher levels of anxiety or depression symptoms. There is some evidence that prescription of antipsychotics to help-seeking CHR-P is related to the severity of positive symptoms and greater impairment of global functioning (Zhang et al., Reference Zhang, Raballo, Zeng, Gan, Wu, Wei and Wang2022). Although we can surmise that the prescription of BDZ, especially when concomitant to the prescription of antipsychotics or antidepressants, might be related to greater severity at presentation, the lack of available information impedes a direct meta-analytical testing. Moreover, we could not test the publication bias with adequate statistics, since the number of studies was less than ten. Nevertheless, since the studies were observational and were not specifically conducted to analyze the effects of BDZ on the risk of transition to psychosis in CHR-P help-seekers, we are reasonably confident that publication bias is unlikely to substantially affect the results. Finally, the geographical distribution of the retrieved studies may limit the generalizability of the findings, although such distribution it is reflective of the differential compliance to transparency standards in reporting baseline pharmacological exposures across studies in the field (i.e. as specified in the methods section, reporting raw data on BDZ baseline exposure in relation to the transition outcome was a necessary pre-requisite for inclusion in the current meta-analysis).
Conclusions
Despite the limitations due to the suboptimal reporting of medication exposure in published CHR-P studies, this study indicates that similarly to antipsychotic and antidepressant baseline exposures, also exposure to BDZs is not irrelevant with respect to the prognostic risk of transition to psychosis. Specifically, baseline exposure to BDZ in newly enrolled CHR-P is associated with an enhanced risk of imminent transition to psychosis. Future research should try to deconstruct the causal factors leading to this macroscopic meta-analytic effect. Such causal deconstruction would increase treatment appropriateness and precision, and hopefully facilitate an overdue, concrete step forward towards precision in the field.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723002180
Acknowledgement
None.
Financial support
None.
Competing interest
The authors have declared that there are no conflicts of interests in relation to the subject of this study.






