Introduction
Humification is a global process that transforms organic material in nature. Humic substances (HSs) are the most stable forms of organic matter in terrestrial and aquatic ecosystems. They serve as a global accumulative matrix of carbon. These macromolecules have a complex structure (Kleinhempel Reference Kleinhempel1970), and their chemical properties are influenced by environmental factors such as climate, biochemical activity, landscape, geological characteristics and the botanical composition of the precursor materials. The most abundant and mature fraction of HSs is humic acids (HAs).
The Antarctic environment is among the least explored in terms of extreme natural habitats for life, and thus also for the decomposition of biological remains. While some studies (Beyer et al. Reference Beyer, Sorge, Blume and Schulten1995, Bonivata et al. Reference Bonivata, Braguglia, DePaolis, Petronino and Schinina1996, Abakumov Reference Abakumov2010) have focused on the organic matter in Antarctic soils, demonstrating that HAs can be isolated from soil samples for instrumental analysis (Calace Reference Calace, Campanella, Depaolis and Petronio1995, Chukov et al. Reference Chukov, Abakumov and Tomashunas2015), it is also important to note that lakes represent significant oases of life within the polar continent, offering a different perspective on biological activity and decomposition. The number of articles focused on HSs in lakes is limited, and they focus mainly on aquatic fractions of fulvic acids (FAs; McKnight et al. Reference McKnight, Aiken and Smith1991, Reference McKnight, Andrews, Spaulding and Aiken1994, Aiken et al. Reference Aiken, McKnight, Harnish and Wershaw1996). Studying HSs in lake sediments is crucial for understanding the full humification/mineralization process of organic matter in Antarctic ecosystems and adding to our knowledge of the global carbon cycle on Earth.
The purpose of this work is to conduct geochemical research on HAs isolated from lake sediments in West Antarctica (King George Island and Marie Byrd Land). The tasks include examining the elemental composition and molecular structure of sediment HAs and identifying the humification conditions in different Antarctic lakes.
Study area
King George Island (62°12′S, 58°58′W) is the largest island in the South Shetland archipelago. It has a polar tundra climate, with an average annual temperature of -2.3°C (https://www.eduardo-frei.climatemps.com). The island is mostly covered by glaciers, with only one-fifth of the territory being free from glaciation, including the Fields Peninsula.
There are ~60 lakes on the peninsula, which were formed by glacial erosion (Watcham et al. Reference Watcham, Bentley, Hodgson, Roberts, Fretwell and Lloyd2011). These lakes are mostly shallow, with an area of < 0.5 km2, except for the larger bodies of water such as lakes Kitezh, Long and Glubokoe, also known as Artigas. The lakes are fed by precipitation and melted glacial waters. During the spring snowmelt, numerous streams form that feed the lakes. By autumn, the melting stops, and many small reservoirs dry up. The dominant ions in the lake water are Na+ and Cl- (Skorospekhova et al. Reference Skorospekhova, Fedorova, Chetverova, Alekseeva, Verkulich, Ezhikov and Kozachek2016). All of these water bodies are covered with ice in winter, and the small reservoirs freeze to their bottom. The ice sheet breaks up in January–February. The local bedrock, consisting of basalts and andesite, is the main source of sediments in the lakes on the Fields Peninsula (Alfonso et al. Reference Alfonso, Vasquez, Hernandez, Mora, Handt and Sira2015, Medeiros Galvão et al. Reference Medeiros Galvão, Vieira, da Rosa, Petsch, Ferreira, Sandes de Oliveira and Cardia Simões2020). Due to the sparse vegetation on the island (Carvalho et al. Reference Carvalho, Costa and Pereira2009), the precursors of humification in lake sediments are mainly moss-lichen and algae residues. In this study, 15 lakes on the Fields Peninsula were examined, and their descriptions are provided below. It should be noted that the lake names used in this study are not official and follow the local toponymy.
Lake 1 (62°10′57.03″S, 58°51′58.64″W) is a small, shallow water body located on the edge of the glacier. The maximum water level (0.3–0.5 m) is reached during periods of intensive glacial melting. The banks are rocky, with rare areas covered in moss and lichen vegetation. In winter, the lake freezes to the bottom.
Lake Glubokoe (Artigas; 62°11′05.5″S, 58°54′36.91″W) is the deepest water body on the peninsula, with a maximum depth of 18.3 m. The lake's shores are steep and composed of volcanic bedrock. In the coastal part of the lake's basin, the bottom is rocky, with sandy-silt deposits predominating at depths > 1 m.
Lake Norma (62°11′20.6″S, 58°55′39.55″W) is a small, shallow water body with a maximum depth of 1.5 m. The lake's shores are flat and covered with basalt debris, with little vegetation present.
Lake Mirazh (62°11′33.88″S, 58°57′14.68″W) has a maximum depth of 6.7 m in its central part. The banks are steep and composed of volcanic rocks, with some snowfields present. In the western part of the lake's basin, there are thickets of underwater mosses Drepanocladus longifolius (Amblystegiaceae; Li et al. Reference Li, Ochyra, Wu, Seppelt, Cai, Wang and Li2009).
Lake Slalomnoe (62°11′31.09″S, 58°57′29.79″W) has a maximum depth of 3 m. The banks are steep and composed of basaltic rocks, with rare hummocks of moss-lichen vegetation present. Underwater moss debris was found on the surface of sediments in the coastal part of the lake, up to a depth of 1.5 m.
Lake Srednee (62°11′35.72″S, 58°57′36.57″W) has a maximum depth of 5.8 m. The lake's shores are low and covered with basalt debris, with dense hummocks of moss present.
Lake Kitezh (62°11′36.66″S, 58°58′00.07″W) is located near Bellingshausen Station and has a maximum depth of 11.5 m. The shores are low and composed of clayey soils with fragments of volcanic bedrock. Moss-lichen vegetation is abundant along the shore, with rare small hummocks of higher vascular plants Deschampsia antarctica (Abakumov Reference Abakumov2010). The majority of the lake's bottom is covered with thickets of underwater moss D. longifolius (Li et al. Reference Li, Ochyra, Wu, Seppelt, Cai, Wang and Li2009).
Lake Bird (62°11′19.18″S, 58°58′08.01″W) is a small, shallow water body with a maximum depth of 1.5 m. At the time of sampling, the water column in the lake had low transparency due to a large amount of green filamentous algae. The lake's shores are low and composed of clayey soils with fragments of volcanic bedrock, with abundant moss-lichen vegetation present. The stones on the shore are covered with decaying debris of moss and algae.
Lake 9 (62°11′20.05″S, 58°58′57.53″W) and Lake 11 (62°11′18.33″S, 58°59′06.39″W) are small water bodies with maximum depths of 0.5 m. The shores are composed of swampy clayey soils with bedrock debris, and the stones around the lakes are covered with pink and green films of biogenic mats. These lakes freeze to their bottom in winter.
Lake 10 (62°11′11.97″S, 58°57′14.58″W) is small water body with the maximum depth of no more than 1.5 m. The shores are flat and composed of clayey soils with bedrock debris.
Lake Penguin (62°12′03.29″S, 58°58′19.03″W) is a small, shallow water body with a maximum depth of 2 m. The shores are low and composed of clayey soils with fragments of volcanic bedrock. The majority of the lake's bottom is rocky, with clayey-silt sediments found only in the western part of the lake. The shores are littered with household waste due to the close proximity of scientific stations and the airport.
Lake Long (62°12′21.93″S, 58°57′57.85″W) is one of the largest lakes on the peninsula, with a maximum depth of 4.3 m. The banks are steep and composed of basaltic rocks, with rocky and decaying debris of mosses and algae present in the coastal part of the basin. Pink films of biogenic mats can be found on the stones around the lake.
Lake Jurasico (62°13′29.41″S, 58°59′57.62″W) and Lake Geographic (62.13′27.49″S, 59.00′14.9″W) are situated in the southern region of the Fields Peninsula. The precise maximum depths of these lakes have not yet been determined. The shores are adorned with decomposing debris of mosses and algae, while pink and green biogenic films can be observed on the stones surrounding the lakes.
Mary Byrd Land (74°45′S, 136°48′W) is located in West Antarctica on the eastern shore of the Ross Sea (Fig. 1). The ice-free land area around Russkaya Station is ~2.2 km2 and consists of two peninsulas. The terrain is composed of gabbroids, which are dissected by veins and dikes of basic, intermediate and acidic composition, running from north to south and from west to east (Tkacheva et al. Reference Tkacheva, Mikhalsky, Sushchevskaya, Kunakkuzin, Skublov and Sergeev2018). The area is characterized by high wind speeds. According to meteorological observations at Russkaya Station from 1980 to 1990, the average long-term number of days with wind speeds > 15 m/s in the area was 264, and for winds > 30 m/s this figure was 136 days. Gusts of wind reached a maximum speed of 75 m/s in 1986. Stable snow cover does not form due to the constant strong eastern winds. The coldest months at the station are July–August (-20°C), and the warmest months are December–January (-2°C). The average annual precipitation is 166 mm. Vegetation cover on the peninsulas is sparse, with only scattered patches of mosses and lichens. In 2022, during seasonal limnological fieldwork, 17 previously unknown lakes were discovered in this area (Demidov & Guzeva Reference Demidov and Guzeva2022). The sediments in most of these lakes consist of boulders and rubble, with silty sediments only found in Lake Humic.

Figure 1. The locations of the studied lakes in West Antarctica (King George Island and Marie Byrd Land).
Lake Humic (74°45′S, 136°49′W) is situated 700 m from the shoreline. Unlike most other water bodies in the area, the lake is almost entirely free of ice in February. It has a maximum depth of 1.5 m, and its shores are rocky with no vegetation in sight. The bottom of the lake is coated with black bacterial mats.
Materials and methods
Sampling procedure
The fieldwork was conducted in February 2022 when all of the lakes were free of ice cover. Physicochemical parameters of water (salinity, pH, Eh) and sediments (pH, Eh) were measured immediately after sampling using a portable Milwaukee Instruments (USA) MW400 analyser. Surface sediments (0–10 cm) were collected from five sites within the basin of each lake using a Van Veen grab. The field description of the sediments included the following characteristics: colour, granulometric composition, smell and presence of inclusions, plant debris and gas bubbles. Additionally, a sample of decomposed plant debris (a mixture of mosses, lichens and algae) was taken from the surface ground and stones of the shores of Lake Bird.
Laboratory analysis
The total content of dispersed organic matter (DOM, mass %) was analysed by digesting absolute dried samples (sieved to 0.2 mm) at 550°C for 5 h. The analyses were conducted on five samples from each lake, with each sample being measured in duplicate to calculate an average value. A difference of 15% or less between the duplicate values was considered acceptable.
For the extraction of HAs, integral sediment samples from each lake and plant debris samples from the shore of Lake Bird were air-dried at 25°C, ground and passed through a 2 mm sieve to remove large plant debris. The samples were then processed according to the standard method of the International Humic Substances Society (Swift Reference Swift, Sparks, Page, Helmke and Loeppert1996).
Elemental analysis of HAs (C, H, N % with correction for water and ash content) was conducted using the Euro EA3028-HT elemental analyser (EuroVector, Italy) at a combustion temperature of 1000°C. The measurement error for C was 0.3%, and for N and H it was 0.2%. The results were corrected for water and ash content, and the oxygen content was calculated according to the difference between the total mass of the sample and the gravimetric concentration of C, N, H and ash. All measurements were performed in duplicate, with a difference of 15% or less between the duplicate values being considered acceptable. The average value for each sample was then calculated.
The molecular structure was examined using solid-state cross-polarization (CP) magic-angle-spinning (MAS) 13C nuclear magnetic resonance (NMR) spectroscopy on a Bruker Avance III 400WB NMR spectrometer (Bruker Corporation, USA) with a 4 mm ZrO2 rotor. The magic angle (54.7°) spinning frequency was 12.5 kHz, the operating frequency was 100 MHz, the contact time was 2 ms, the delay time was 3 s and the number of scans was 2048.
Data obtained for processing
Mass percentages (mass %) of C, H, N and O were converted to mole percentages (mole %) to determine the role of elemental atoms in the construction of HA molecules. Atomic fractions were also used to calculate H/C and O/C ratios.
Quantitative treatment was carried out for numerical integration of the areas of the 13C-NMR spectra using the software MestreNova 8.0 and MagicPlot. The percentages of the following types of C in molecular fragments were calculated in correspondence with their spectral position: C,H-substituted aliphatic fragments (0–47 ppm); methoxyl and O,N-substituted aliphatic fragments (47–60 ppm); aliphatic fragments doubly substituted by heteroatoms (including carbohydrate) and methine carbon of ethers and esters (60–110 ppm); C,H-substituted aromatic fragments (110–144 ppm); O,N-substituted aromatic fragments (144–160 ppm); carboxyl groups, esters, amides and their derivatives (160–185 ppm); and quinone groups and groups of aldehydes and ketones (185–200 ppm; Yao et al. Reference Yao, Zhang, Han, Han, Mao and Zhang2019, Guzeva Reference Guzeva2022). The parameter ALH,R + ARH,R (the sum of the regions of 0–47 and 110–144 ppm) provided information regarding the amphiphilic properties of the HAs (molecular hydrophobicity; Lodygin et al. Reference Lodygin, Beznosikov and Vasilevich2014). The degree of organic matter humification (decomposition) was evaluated using the C,H-al/O,N-al ratio (Pedersen et al. Reference Pedersen, Simpson, Bockheim and Kumar2011).
Typical absorption bands of HAs in the Fourier-transform infrared (FTIR) spectra of all studied samples were identified according to previous research (Chen et al. Reference Chen, Leboeuf, Choia and Gua2002, Guzeva Reference Guzeva2022).
Results
Hydrochemical and geochemical characteristics of the studied lakes
Table I presents the physical and chemical parameters of the water and sediments in the studied lakes. The lakes' waters on King George Island are fresh and have a pH close to neutral, with a positive redox potential (Eh). The sediments consist mainly of silt and aleurite (fine sand). The organic matter content (DOM) in the sediments did not exceed 10%, and the pH ranged from weakly acidic to neutral. The majority of the lakes had a positive Eh, with the exception of four lakes (Table I). Lake Humic (Marie Byrd Land) had the highest salinity (3 g/l) of water among the studied lakes. The sediments of this lake were enriched with organic matter and had an acidic pH.
Table I. Physical and chemical parameters of the water and sediments of the studied lakes in West Antarctica (King George Island and Marie Byrd Land).

a Means of five samples are presented.
b The results of the isolation of the humic acid fraction: + = humic acids have been extracted; - = humic acids cannot be extracted in quantities sufficient for instrumental study.
DOM = dispersed organic matter; Eh = redox potential; TDS = total dissolved salinity.
Humic acid extraction
The alkali-soluble fraction (HAs) has been extracted in sufficient quantities for instrumental analysis from four lakes on King George Island, as well as one sample of plant residue (Table I). The HAs were characterized as having a light brown colour. However, from the sediments of the other studied lakes on the island, only a light yellow solution of FAs could be obtained, as the HAs did not precipitate even under low-pH conditions. In contrast, the HAs from Lake Humic had a dark brown colour and visually appeared to precipitate in greater quantities compared to HAs from the lakes on King George Island.
Elemental analysis of humic acids
The data obtained on the elemental composition of HAs extracted from plant debris and lake sediments in West Antarctica are presented in Table II. The HA molecules found in plant debris, which are precursors to humification, contained the lowest mass percentage of carbon and the highest amount of oxygen. The number of oxygen atoms (measured in mole %) was almost twice that of carbon atoms. In HAs isolated from the lake sediments, the level of oxygen atoms decreased, while the degree of carbonization of molecules increased.
Table II. Elemental composition of humic acids (ash- and water-free weights) extracted from plant debris and lake sediments of West Antarctica (King George Island and Marie Byrd Land).
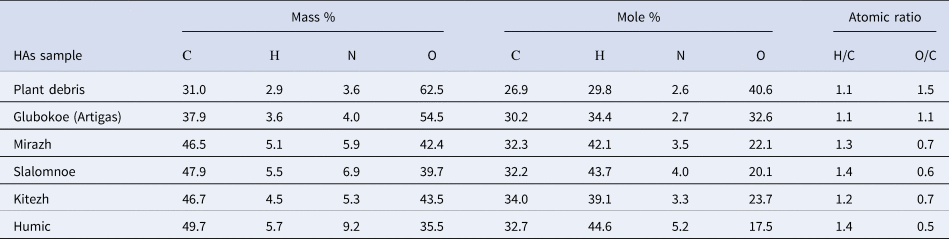
According to the H/C atomic ratio values (degree of saturation of molecules), the studied HAs were closer to aliphatic cycloparaffins than to aromatic compounds. The most saturated HAs were those from the sediments of lakes Slalomnoe (King George Island) and Humic (Marie Byrd Land).
CP MAS 13C-NMR spectroscopy of humic acids
The NMR spectra and the percentages of the various molecular fragments are presented in Fig. 2: unsubstituted aliphatic ((CH2)n/CH/C); methoxyl and O,N-substituted aliphatic (NCH/OCH3); carbohydrate and methine carbon of ethers and esters (OCH/OC/O-CH-O/O-C-O); unsubstituted aromatic (aromatic C-C/C-H); O,N-substituted aromatic (aromatic C-O); carboxyl groups, esters, amides and their derivatives (COO/N-C=O); and quinone, aldehydes and ketones (RC(=O)R'/RCHO). Aliphatic carbon species were the dominant structures in all of the studied HAs samples. The percentage of aromatic structures varied from 2% in HAs found in plant debris to 19% in sediment HAs of Lake Glubokoe (Artigas).
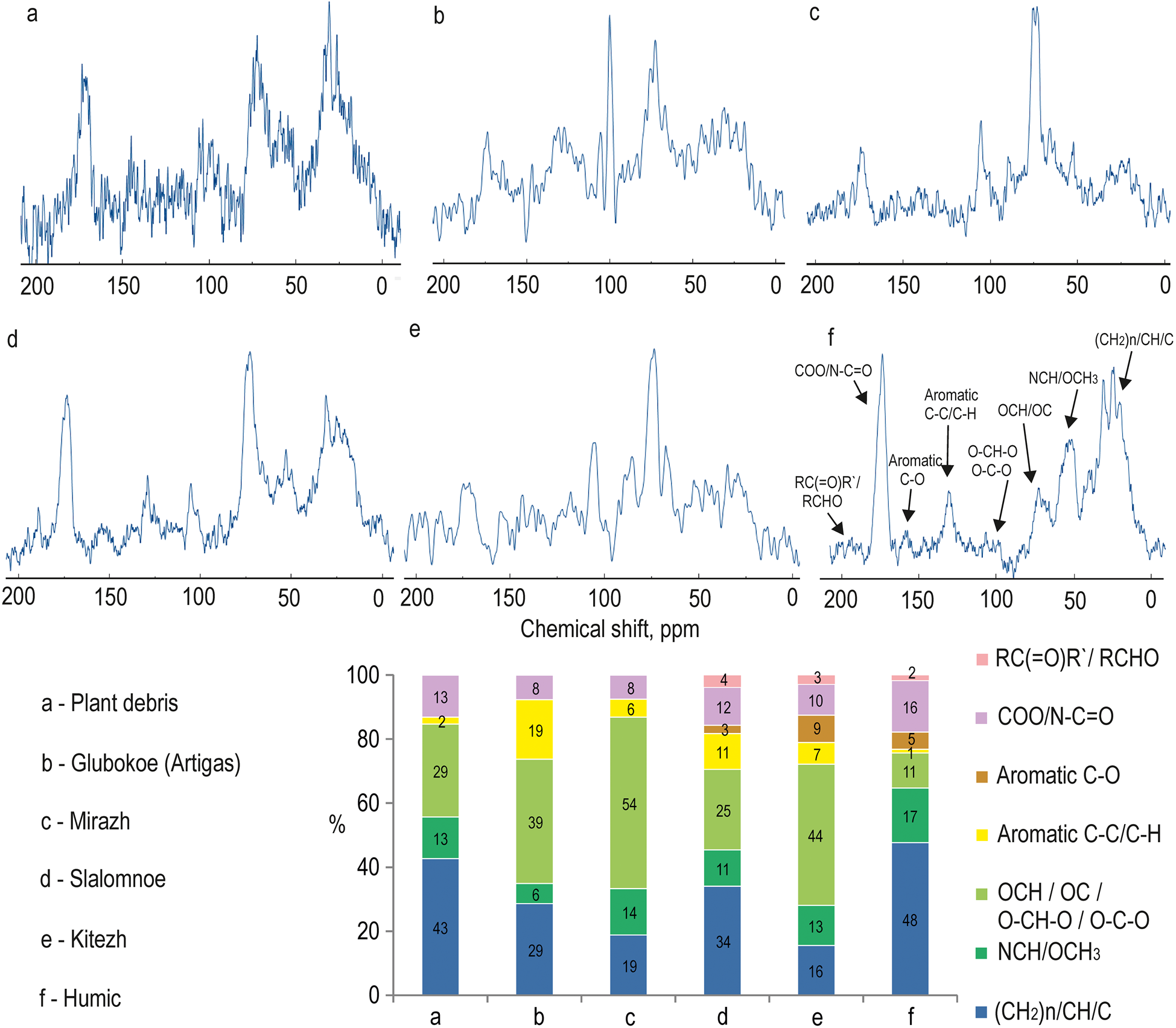
Figure 2. 13C nuclear magnetic resonance (NMR) spectra of humic acids extracted from plant debris and lake sediments of West Antarctica (King George Island and Marie Byrd Land).
Most aliphatic fragments in sediment HA samples from King George Island were O,N-substituted. By contrast, the content of unsubstituted aliphatics is higher than O,N-substituted aliphatics in the sample from Lake Humic (Marie Byrd Land). The percentages of these structures were consistent in the sample from plant debris.
The functional O/N-containing groups (carboxyl, esters, amides and their derivatives) in the region of 160–185 ppm were detected in all of the studied HA samples. Quinone, carbonyls of aldehydes and ketones, hydroxyls of alcohols and phenolic fragments were found in samples of sediment HAs from lakes Slalomnoe, Kitezh and Humic. However, the contents of the groups were no more 4%.
FTIR spectroscopy of humic acids
The peaks provided by valent (2920 and 2860 cm-1) and deformation (1460–1440 cm-1) stretching of aliphatic fragments were observed in all HA samples (Fig. 3). The band in the region of aromatic vibrations (1700–1590 cm-1) was detected in all of the studied samples, with the most intense peak observed in the sample from Lake Humic. However, it should be noted that the stretching of carbonyls and amides could also absorb in same spectral area. Hydrogen-bonded OH-groups provided wide bands in the range of 3500–3300 cm−1 and low peaks at 1270–1220 and 1170–1040 cm-1. The peaks (in samples from plant debris and Lake Glubokoe) or shoulders (in lakes Mirazh, Slalomnoe, Kitezh and Humic) at 1715–1710 cm-1 were associated with C=O stretching of -COOH and ketonic carbonyls. Deformation vibrations of N-H and C=N provided clear peaks at 1540–1510 cm-1 range. This peak is most intense in the sample from Lake Humic. Valent stretching of -OH from alcohols and carbohydrates was detected in the 1175–1000 cm-1 spectrum area. C-O groups of primary alcohols produced clear peaks in the 1075–1013 cm-1 range.
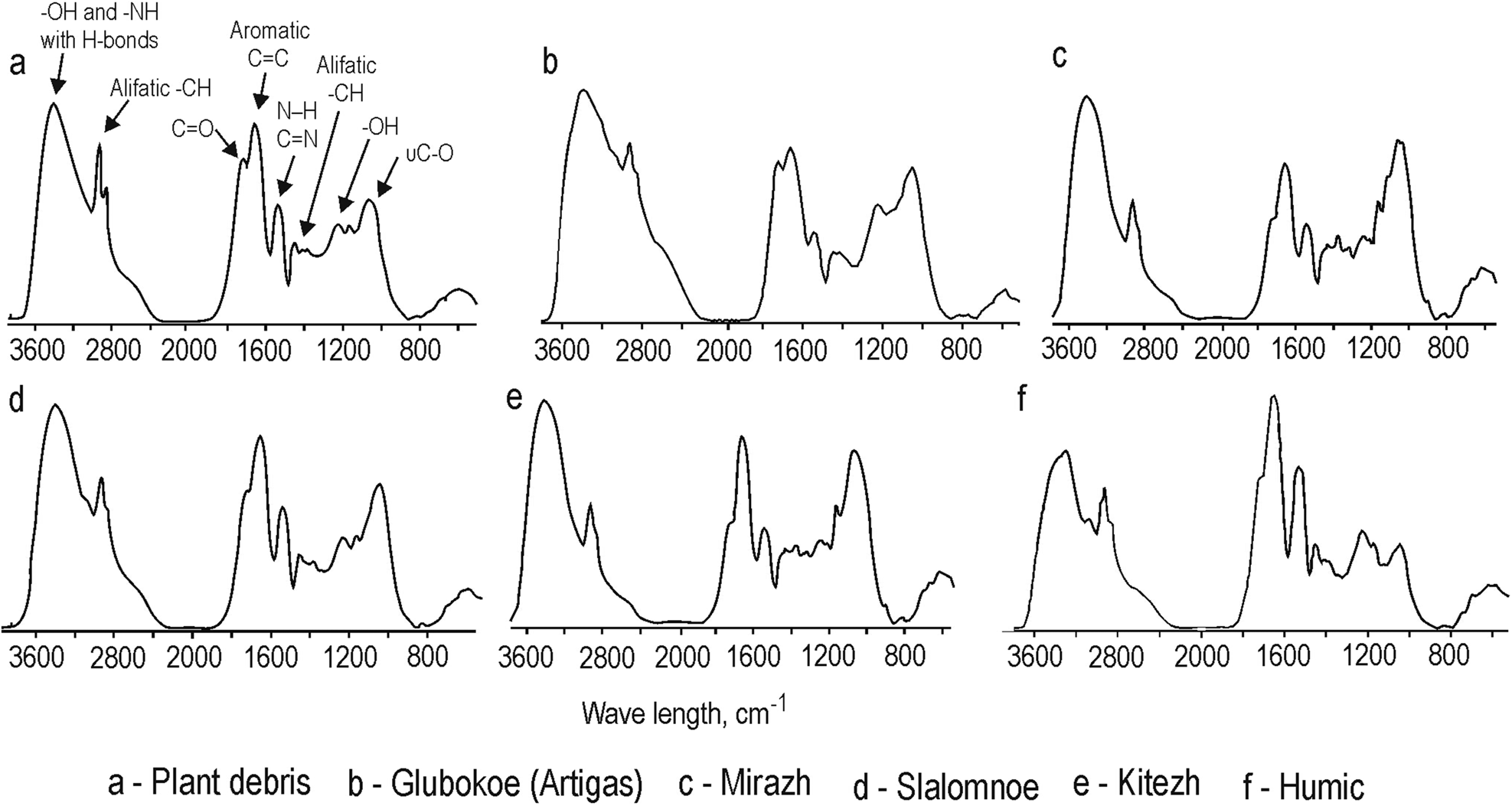
Figure 3. Fourier-transform infrared (FTIR) spectra of humic acids extracted from plant debris and lake sediments of West Antarctica (King George Island and Marie Byrd Land).
Discussion
Chemical characterization of humic acids
The compounds under study exhibit a high degree of molecular complexity and composition. The spectral analyses yield consistent results, providing sufficient evidence to determine the dominant patterns of HA structure. These findings can be compared to other studies of HSs in the natural environments of Antarctica. The HAs found in the sediments display a saturated aliphatic structure, a characteristic also observed in HSs from other sources such as water and soil in Antarctica (McKnight et al. Reference McKnight, Aiken and Smith1991, Calace et al. Reference Calace, Campanella, Depaolis and Petronio1995, Abakumov Reference Abakumov2010). Statistical analysis (Fig. 4) showed that for HAs (both soils and sediments) and aquatic FAs the H/C ratio was in the range of cycloparaffins and varied from 1.1 to 1.9.

Figure 4. The H/C and O/C atomic ratios (van Krevelen diagram) for humic substances from Antarctica. FA = fulvic acid; HA = humic acid.
1Humus-forming substances (penguin guano, lichens, mosses, algae) according to Abakumov (Reference Abakumov2010).
2Fulvic acids from the waters of Lake Fryxell and Lake Hoare (McKnight et al. Reference McKnight, Aiken and Smith1991).
3Humic acids from the soils of Oates Coast (Calace et al. Reference Calace, Campanella, Depaolis and Petronio1995).
4Humic acids from the soils of Schirmacher Oasis, Lindsay Island, King George Island (Chukov et al. Reference Chukov, Abakumov and Tomashunas2015).
The degree of oxidation (O/C) of the studied HA molecules is similar to that of soils found in Schirmacher Oasis (Fig. 1), Lindsay Island and King George Island (Chukov et al. Reference Chukov, Abakumov and Tomashunas2015), but it is relatively high compared to the soils of Oates Coast (Fig. 4; Calace et al. Reference Calace, Campanella, Depaolis and Petronio1995). The high oxygen content of HAs in Antarctic lake sediments can be attributed to the presence of a significant amount of oxygen-containing fragments, such as methoxyls and polysaccharides, in their structure (Figs 2 & 4).
The molecules of humification precursors (such as penguin guano, lichens, mosses and marine algae) and HAs from plant residues are more saturated and oxidized than sediment and soil HAs (Fig. 3). Thus, the transformation of biological material into HSs in Antarctica's natural environment is mainly accompanied by dehydration and a slight increase in aromaticity.
Humification in the lake sediments
The humification process in the lakes of King George Island takes place under freshwater conditions with pH values close to neutral. During the ice-free period, the water column is sufficiently enriched with oxygen, resulting in positive Eh values. The sediments of the studied lakes contain a small amount of organic matter (mineral silts). Weakly reducing (anoxic) environments are observed in the sediments from four lakes. The lowest Eh value was recorded in the sediments of Lake Slalomnoe, where a large number of odourless gas bubbles, probably methane, were also observed in the samples. The precursors of humification in the island's ecosystems are the remains of sparse vegetation, such as mosses, lichens, and algae. The process of maturation of HSs in lake sediments is associated with dehydration of precursor molecules, resulting in a slight increase in the contents of aromatic fragments in their structures. It is important to note that the FA fraction significantly predominates in the composition of HSs in lake sediments. The most mature fraction (HAs) can only be isolated in sufficient quantities for instrumental analysis from some water bodies. The predominance of the fulvic type of humus has been previously observed in the HSs found in the water of Antarctica's Carezza Lake (Victoria Land, Ross Sea coast; Campanella et al. Reference Campanella, Petronio, Braguglia, Cini and Degli Innocenti1995). Thus, the low content of the HA fraction in HSs and the aliphatic structure suggest a low degree of maturity of HSs in the lake sediments of King George Island. This is due to weak biochemical activity, which leads to the accumulation of slightly modified peptide and carbohydrate chains in the structure of the HAs. This is supported by results from previous studies on soils and sediments in cold climatic zones (Lodygin et al. Reference Lodygin, Beznosikov and Vasilevich2014, Polyakov & Abakumov Reference Polyakov and Abakumov2020). In these environments, mineralization of organic matter is more prevalent than humification.
Lake Humic (Marie Byrd Land) has a brackish composition and a neutral water pH. The sediments of the lake do not freeze completely in winter due to the formation of cryopeg in the bottom layer of the water. Full-fledged humification of organic material occurs in Lake Humic. At the bottom of the lake, bacterial films decompose and accumulate, serving as the main precursors for humification. The sediment conditions are acidic and weakly reducing. The HAs are primarily composed of aliphatic molecules, but the proportion of O/N-substituted aliphatics is lower compared to samples from other lakes studied on King George Island.
Figure 5 illustrates the resistance of the studied HAs to biochemical oxidation. Polysaccharide chains are the most easily accessible components of HAs for processing by microorganisms (Zherebker et al. Reference Zherebker, Podgorski, Kholodov, Orlov, Yaroslavtseva and Kharybin2019). The HAs from Lake Humic exhibit the most stable structure (Fig. 5). By contrast, the HAs from the lakes of King George Island do not mature significantly due to the presence of dense underwater moss growth at their bottom. This suggests that HSs are rapidly broken down by microorganisms and mineralized in environments with high levels of photosynthesis and oxygen.

Figure 5. Diagram of humification degree of humic acids extracted from the lake sediments of West Antarctica (King George Island and Marie Byrd Land).
Conclusion
This work is the first to present an investigation of the elemental composition and molecular structure of the alkaline-soluble fraction of HSs isolated from lake sediments of Antarctica (King George Island and Marie Byrd Land). The studied compounds contained specific molecular fragments and can be identified as HAs. Thus, full-fledged humification processes occur in the sediments of some Antarctic lakes. However, the HSs of the studied lakes were found to have a very low degree of maturity and were non-resistant to mineralization. The structural properties of the studied HAs were typical of HSs formed in the cold climatic conditions of Arctic and Antarctic environments. This work provides information on the natural environment of the poorly explored region of Marie Byrd Land in Antarctica. Moreover, this research is valuable for understanding the full chain of organic matter transformation processes in Antarctic ecosystems and the global carbon cycle on Earth.
Acknowledgements
The fieldwork in this study was carried out as part of the seasonal work of the 67th Russian Antarctic Expedition. The author thanks her colleagues Zakhar Slukovskii and Nikita Demidov for their assistance in the sampling procedure.
Financial support
This research is supported by the Russian Science Foundation project no. 22-27-00131 (chemical analysis) and Governmental Order to the Institute of Limnology RAS - St Petersburg Federal Research Center of RAS No. FFZF-2024-0002 (interpretation and statistical processing of data).
Competing interests
The author declares none.
Data availability
The datasets generated and/or analysed during the current study are available from the author on reasonable request.
Author contribution
AVG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.










