Introduction
Spinal instrumentation is performed routinely for internal stabilization to promote osseous fusion in traumatic, degenerative, metabolic, and neoplastic spinal pathologies. With an aging population, the North American burden particularly of degenerative and osteoporotic spinal injuries is increasing, with tremendous societal and economic costs.Reference Cadarette and Burden 1 – 4 Instrumentation misplacement can result, acutely, in injury to adjacent neurovascular structures and, in the long term, to hardware failure and non-union from poor load-bearing properties.Reference Xiao, Miller and Sabharwal 5 , Reference Acikbas, Arslan, Tuncer, Matge and Muciejczak 6 The placement of spinal instrumentation is traditionally performed freehand, or with guidance from intra-operative X-rays or fluoroscopy resulting in significant radiation exposure to operating room (OR) personnel and/or the patient.Reference Villard, Ryang and Demetriades 7 , Reference Nelson, Monazzam, Kim, Seibert and Klineberg 8 Three-dimensional computer-assisted navigation (CAN) has been shown to improve the accuracy of screw placement and reduce surgeon radiation exposure, across all spinal levels.Reference Nelson, Monazzam, Kim, Seibert and Klineberg 8 – Reference Mirza, Wiggins and Kuntz 12 Emerging evidence supports potentially improved short- and long-term clinical outcomes with the use of spinal CAN, with reduced reoperation for hardware malposition-related complications as well as wound infections.Reference Xiao, Miller and Sabharwal 5 , Reference Luther, Iorgulescu and Geannette 13 , Reference Fichtner, Hofmann and Rienmüller 14 The CAN usage is also cost-effective in high-volume centres.Reference Dea, Fisher and Batke 15 , Reference Sanborn, Thawani and Whitmore 16 However, CAN is used routinely by only 10–15% of spinal surgeons.Reference Hartl, Lam, Wang, Korge, Kandziora and Audige 17 – Reference Schröder and Wassmann 19 A worldwide survey of spinal surgeons, representing predominantly Europe, Asia, and Latin America, revealed multiple barriers to CAN adoption, principally cost, lack of training, workflow disruption, and unproven clinical benefit.Reference Hartl, Lam, Wang, Korge, Kandziora and Audige 17 The potential benefit of intra-operative CAN for trainee education is also poorly represented in assessments of spinal CAN utility.Reference Sundar, Healy, Kshettry, Mroz, Schlenk and Benzel 20 – Reference Podolsky, Martin, Whyne, Massicotte, Hardisty and Ginsberg 26
Given differences in health care economics in Canada relative to the United States and Europe, with potentially different barriers to CAN adoption, we propose here to answer the following questions: First, what is the current pattern of spinal CAN utilization across a cohort of Canadian institutions and practitioners? Second, what is the utility of intra-operative spinal CAN for trainee education? By answering these questions, we hope this study identifies barriers to adoption of spinal CAN specific to the Canadian health care system and proposes solutions to mitigate them, in order to facilitate translation of a technology shown to improve short- and long-term outcomes for Canadian patients undergoing spinal instrumentation.
Methods
Study Design
Assessment of temporal trends in spinal CAN utilization in academic and community neurosurgical and spinal centers was performed by retrospective review of a prospectively maintained provincial database of diagnostic and fee codes.
The utility of spinal CAN for trainee education was explored through an online survey, administered to a nationwide cohort of neurosurgical and orthopedic surgical residents and clinical spine fellows.
Database—Patient Selection
The Ontario Health Insurance Plan (OHIP) database was searched through the Institute of Clinical and Evaluative Sciences (ICES), at Sunnybrook Health Sciences Centre, for records from 1 January 2005 to 31 December 2014 (REB# 380-2015). Patients meeting the following criteria were included: ≥18 years of age; undergoing instrumented spinal fusion from either an anterior or posterior approach at any spinal region; or undergoing percutaneous or open vertebroplasty or kyphoplasty. Patients undergoing non-instrumented spinal fusion, or spinal decompression without instrumentation, were excluded.
Database—Data Extraction
All data were extracted from the OHIP database by ICES analysts. Procedures were classified by pathology as trauma, degenerative, deformity, infection, tumor, and vertebroplasty/kyphoplasty, based on a combination of OHIP fee and International Classification of Disease Ninth Revision, Clinical Modification (ICD-9-CM) codes. Within each pathology, procedures were sub-classified by spine region (cervical/thoracic/lumbosacral) and surgical approach (anterior/posterior), using a combination of OHIP fee and ICD-9-CM codes. The use of two-dimensional (2D) or three-dimensional (3D) spinal CAN for each procedure was identified using fee codes (E379/E378).
For each identified procedure, the following demographic data were extracted: patient age, gender, institution type (academic/rural), and surgeon specialty (orthopedic surgery/neurosurgery).
Database—Statistical Analysis
Univariate comparison of categorical variables, including the proportion of procedures undertaken with 2D or 3D CAN, were performed using Pearson χ 2 or Fisher’s exact tests, depending on data distribution, with computation of Pearson’s correlation coefficients. Continuous variables were compared using independent samples t-tests or Mann-Whitney U-tests, depending on data distribution.
Predictors of CAN usage were explored using binary multiple logistic regression modeling, as well as hierarchical mixed-effects logistic regression to account for surgeon specialty and institution type as random effects.
Significance levels for all tests were set at α<0.05. All statistical analyses were performed in SAS (version 9.3; SAS Institute Inc., Cary, NC).
Online Survey
The utility of spinal CAN for Canadian surgical trainees was assessed using a 22-item anonymized online questionnaire distributed through GoogleForms (Appendix A). The survey was disseminated in September 2015 by email to 241 orthopedic surgical and neurosurgical residents across 15 Canadian training programs, as well as 31 clinical adult and pediatric spine fellows. A follow-up request for completion was emailed at 1 month; responses were collected for a total of 4 months.
Responses to questions with multiple-choice ordinal options were converted to ordinal numerical variables for analysis. All other responses were converted to nominal categorical variables. Comparisons between categorical variables were made using Pearson χ 2 tests or Fisher’s exact tests, depending on data distribution. Comparisons between multiple proportions were made using partitioned χ 2 analyses with Bonferroni correction. User comfort with instrumentation techniques with versus without navigation guidance was assessed using Wilcoxon signed-rank tests.
Statistical analyses for the online survey were performed in SPSS (version 21; IBM, Chicago, IL).
Literature Review
A systematic search of the literature on the use of spinal CAN for trainee education was conducted in MEDLINE, limited to primary human studies published in the English language from 2000 to present. Reference lists of key articles were checked to identify additional eligible articles.
Studies were included if the type of navigation technique, training environment, and training task were specified. Outcome measures, whether quantitative or qualitative, were required to be reported. Narrative and systematic reviews were excluded.
Results
Spatio-Temporal Trends in Spinal CAN Usage
A total of 4607 cases of spinal instrumentation were identified in the OHIP database from 2005 to 2014, 35.8% with temporary percutaneous instrumentation (vertebroplasty/kyphoplasty) and the remainder with permanent hardware for fusion (Figure 1). Of the cases 45.9% were performed at an academic institution, with 67.7% of instrumented fusions performed by orthopedic surgeons and the remainder by neurosurgeons.
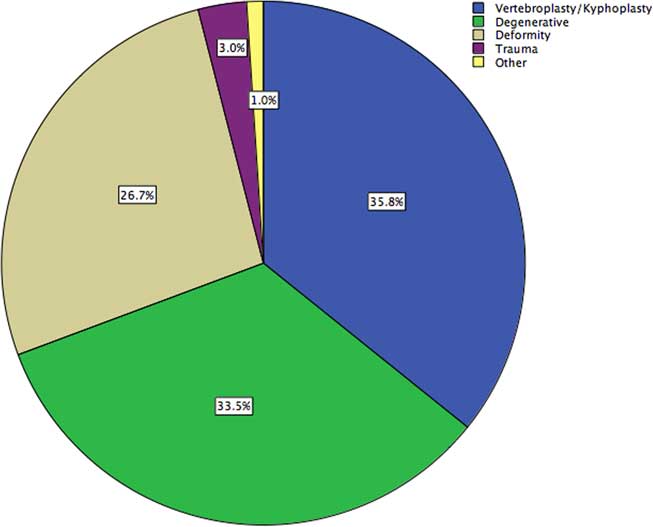
Figure 1 Demographics of a provincial cohort of patients undergoing spinal instrumentation, stratified by pathology.
Intra-operative CAN was used in 14.0% of cases, with 16.8% of instrumented fusions. Navigated cases were guided predominantly by 3D-CAN, with 27.1% using 2D-CAN. In this cohort, CAN was used most frequently for trauma (41.8%, with 32.1% 3D-CAN), followed by degenerative pathologies (19.8%, with 94.6% 3D-CAN) and deformity corrections (2.5%, with 66.7% 3D-CAN). Computer-assisted navigation was used in only 0.5% of vertebroplasties/kyphoplasties. In univariate analysis, CAN was used more frequently in academic institutions (15.9% vs. 12.3%, p<0.001), and by neurosurgeons more than orthopedic surgeons (21.0% vs. 12.4%, p<0.001). Temporal trends in CAN usage are shown in Figure 2.
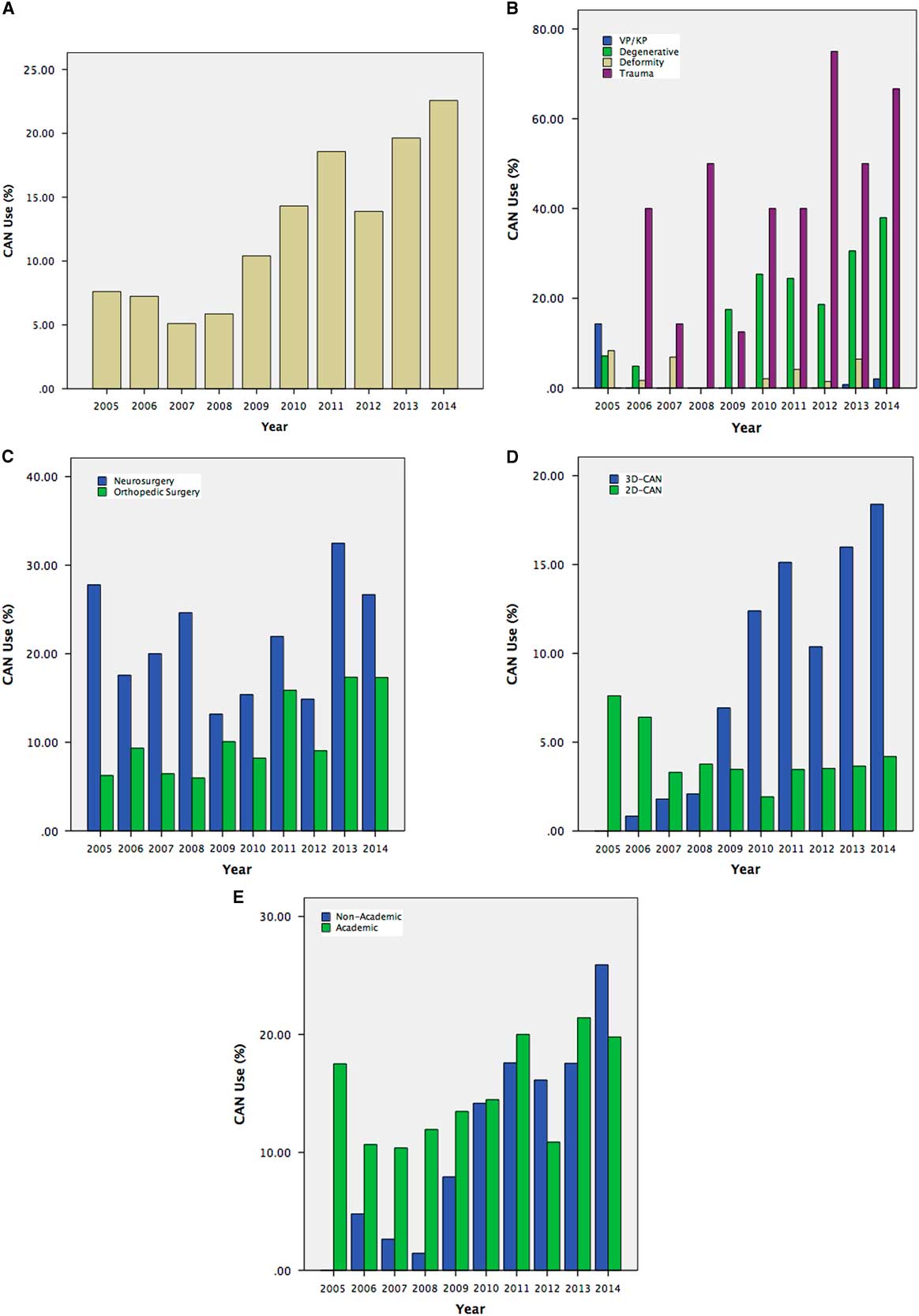
Figure 2 Temporal trends in overall spinal CAN usage (A) for a provincial cohort of patients undergoing spinal instrumentation, stratified by pathology (B), surgeon specialty (C), 2D versus 3D-CAN (D), and by institution type (E). Vp=vertebroplasty, Kp=kyphoplasty.
In hierarchical logistic regression, accounting for patient age, gender, pathology, and surgical approach as fixed effects, and individual institutions and surgeons as random effects, surgeon specialty and institution type were not independently associated with increased CAN usage. The intra-class correlation coefficients for individual institutions and surgeons were 24% and 64%, respectively. That is, the majority of variation in CAN usage is based on hospital and surgeon individual preference.
Survey of Surgical Trainees—Demographics
Of 272 residents and clinical spine fellows polled, complete responses were obtained from 60, for a response rate of 22.1% (Figure 3). Orthopedic surgery and neurosurgery were represented equally at 50% each. Respondents were located predominantly in Ontario (55.0%), followed by Quebec (20.0%) and Alberta (10.0%); the remaining respondents were located in British Columbia, Saskatchewan, Manitoba, Nova Scotia, and Newfoundland. Non-responders were predominantly in their PGY-1 or PGY-2 years of training.
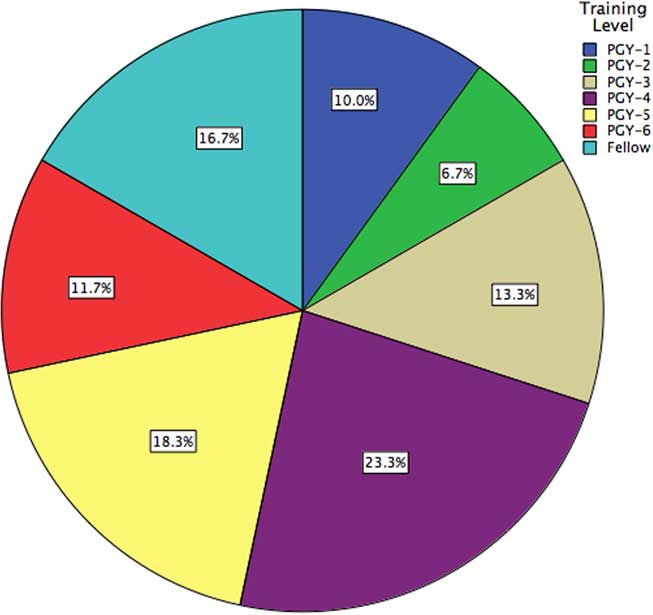
Figure 3 Demographics of a surveyed national cohort of neurosurgical and orthopedic surgical trainees, stratified by training level.
Among surgical residents, the average time spent on a dedicated spine service was 5.50±6.71 months, significantly greater for neurosurgery (7.37±8.62 months) than orthopedics (3.30±1.82 months) (p=0.024).
Utilization of CAN by Trainees
Among trainees 73.3% identified CAN as being available at their institution. Across all case types, CAN was used >40% of the time by only 34.1% of respondents, with no differences between surgical specialties.
In subgroup analyses looking at open fusions, minimally invasive spinal (MIS) fusions, deformity corrections, and revision fusions, CAN was used in >40% of cases by 38.6%, 36.6%, 32.1%, and 38.6% of respondents, respectively, with no differences between surgical specialties (Figure 4). In partitioned χ 2 analysis, there was no significant difference in CAN usage between case types.
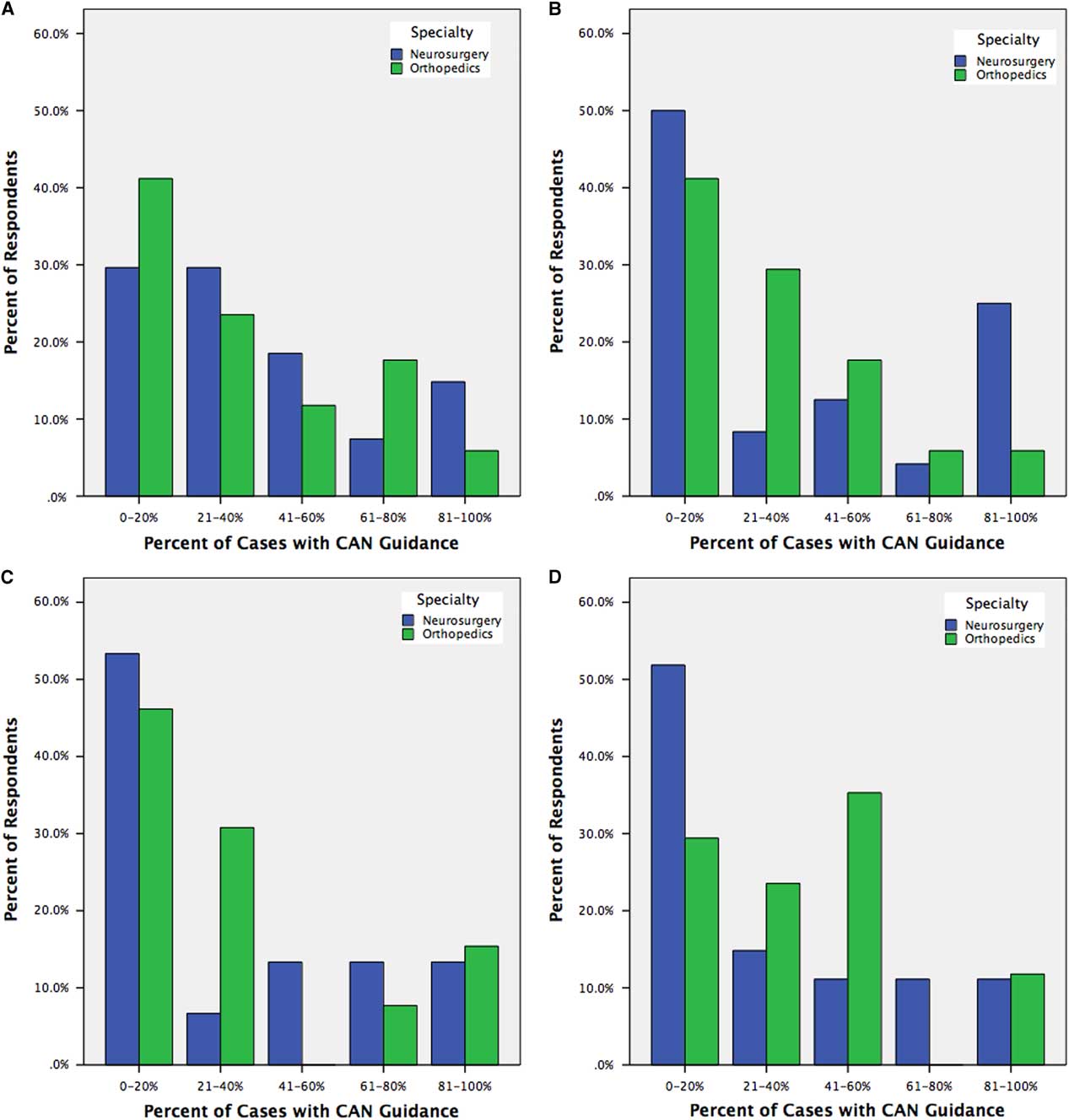
Figure 4 Trainee reporting of CAN usage for open instrumented fusions (A), minimally invasive instrumented fusions (B), deformity corrections (C), and revision instrumented fusions (D), stratified by trainee specialty.
Of residents, 34.1% were identified as being fully capable in the setup and intra-operative use of the CAN system available at their institution, either independently or with minimal supervision, significantly greater among neurosurgical than orthopedic trainees (48.1% vs. 11.8%, p=0.008). Instruction on CAN setup/use was provided by surgical faculty for 75.0% of respondents, by CAN product representatives for 52.3%, by fellows for 22.7%, by senior residents for 20.5%, and by self-teaching for 22.7%.
Impact of CAN on Trainee Proficiency
Self-reported trainee proficiency with instrumentation in the atlantoaxial, subaxial cervical, thoracic, and lumbosacral spine was compared with and without CAN guidance (Appendix A, Questions #13–20). An 11.0% increase in mean proficiency rank (2.93 vs. 2.64, p=0.036) was seen for thoracic pedicle screws with versus without CAN guidance, across all respondents (Figure 5).
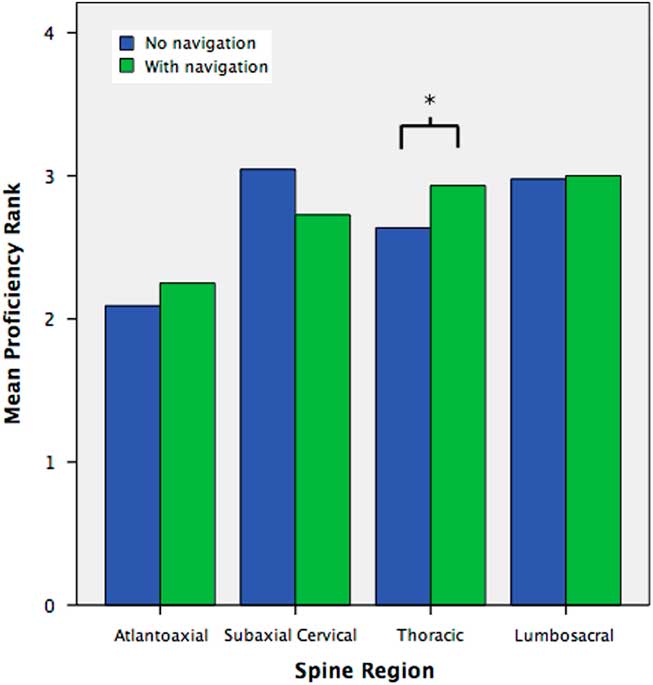
Figure 5 Mean self-reported proficiency rank of trainees for the placement of atlantoaxial, subaxial cervical, thoracic, and lumbosacral instrumentation, with versus without CAN guidance. Proficiency was self-reported as 1=not at all competent; 2=somewhat competent, requiring extensive supervision; 3=very competent, with supervision; 4=fully independent, without supervision. (*) denotes significant difference at p<0.05.
When stratified by specialty, neurosurgical residents reported improved but statistically insignificant gains in proficiency with CAN guidance for thoracic instrumentation (2.85 vs. 2.59, p=0.198), whereas orthopedic surgical residents reported an 18.0% increase in mean proficiency rank with atlantoaxial instrumentation (2.29 vs. 1.94, p=0.014) as well as a 12.9% increase in mean proficiency with thoracic instrumentation, just missing statistical significance (3.06 vs. 2.71, p=0.058).
Literature Review
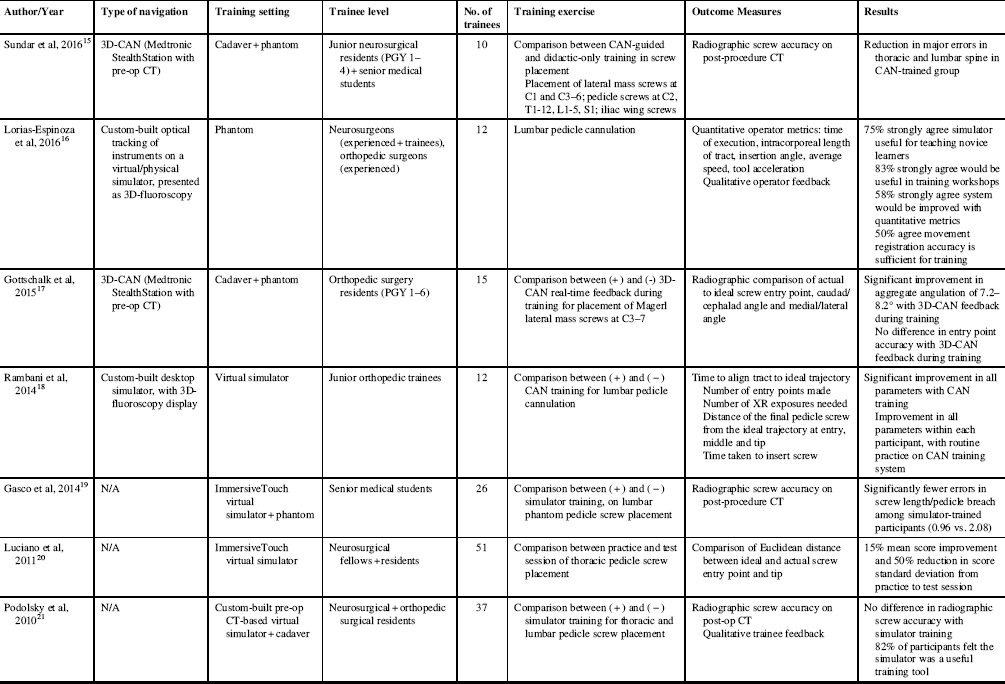
From our initial search for articles pertaining to the use of spinal CAN for trainee education, 53 abstracts were identified. Of these, the full-texts of 14 were reviewed, with the remainder eliminated largely for not focusing on trainee participants. From the literature search, seven studies were identified, all of which were conducted in a virtual reality or cadaveric/phantom setting (Table 1).
Table 1 Summary of the literature on spinal navigation for trainee education
Discussion
The adoption of spinal CAN remains limited by steep learning curves with potentially prolonged operating times initially, and significant workflow disturbances primarily from registration protocols.Reference Hartl, Lam, Wang, Korge, Kandziora and Audige 17 , Reference Choo, Regev, Garfin and Kim 18 , Reference Ryang, Villard and Obermüller 27 , Reference Wood and McMillen 28 Increasing the uptake of a technology proven to improve accuracy and patient outcomes requires an understanding of current practice patterns and barriers to adoption. To our knowledge, our study represents the first to explore the use of spinal CAN in a single-payer health care system.
We show here that spinal CAN is used predominantly by neurosurgeons and in academic institutions. This conclusion is unsurprising given that intra-operative frameless stereotactic CAN was developed originally for intracranial tumor localization.Reference Roberts, Strohbehn, Hatch, Murray and Kettenberger 29 Although surgical technique, beyond anterior versus posterior approaches, was not captured in the database review, our online survey of Canadian surgical trainees revealed that CAN was used equally for open fusions, MIS fusions, revision fusions, and deformity cases. This contrasts with the trend seen in the United States, where CAN appears to be used most often in high-volume MIS practices, and may reflect a lack of deployment of CAN, in Canada, in the settings in which it is clinically most useful.Reference Hartl, Lam, Wang, Korge, Kandziora and Audige 17 Conversely, the relative deficiency of CAN in MIS and deformity procedures in Canada may reflect a relatively lower volume of these cases overall, due in part to prolonged operating times and increased OR radiation exposure with MIS cases compared to equivalent open procedures.Reference Bindal, Glaze, Ognoskie, Tunner, Malone and Ghosh 30 , Reference Funao, Ishii and Momoshima 31 Both issues are addressed by current and emerging CAN techniques; willingness of institutions and practitioners to adopt CAN technology may encourage safer, more efficient, and less invasive spinal procedures for patients.Reference Jakubovic, Guha and Lu 32
Real-time CAN feedback on anatomic landmark identification may also be beneficial for trainees in learning spinal anatomy and nuances of instrumentation. To our knowledge, our study represents the first to explore the utility of CAN intra-operatively for trainee comfort and proficiency in placing instrumentation.
In our online survey, only one-third of residents reported being fully capable of setting up and using a CAN system without or with minimal supervision, greater among neurosurgical than orthopedic surgical trainees. The lack of comfort in CAN use among residents overall is reflected in the similar lack of comfort and training for current faculty, one of the major barriers to adoption that may be addressable through improved practical education at the trainee level.Reference Hartl, Lam, Wang, Korge, Kandziora and Audige 17 , Reference Choo, Regev, Garfin and Kim 18 The relative lack of comfort with CAN for orthopedic surgical trainees compared to their neurosurgical counterparts may be in part due to lack of familiarity with CAN from non-spinal procedures, as well as significantly less time spent on a dedicated spine service. However, the intra-operative use of CAN appears to improve the self-reported proficiency of all trainees, in fact to a greater degree for orthopedic surgical trainees. This is in keeping with the findings of ex vivo laboratory studies.Reference Sundar, Healy, Kshettry, Mroz, Schlenk and Benzel 20 Given that orthopedic surgeons performed most of the instrumented spinal fusions in our retrospectively reviewed Ontario cohort, it may be prudent to increase education in spinal CAN techniques at the trainee level, to improve adoption particularly within the orthopedics community and thereby maximize the potential benefits of CAN. For orthopedic surgeons, as exposure to spine in residency is limited and typically requires a post-graduate fellowship, education in spinal navigation at the fellowship stage is most practical and likely to be of benefit. Most respondents in our cohort reported being instructed on CAN use by their attendings; improvement in trainee CAN education thus requires increased adoption among faculty, by addressing known concerns with CAN such as workflow and registration hindrances.Reference Hartl, Lam, Wang, Korge, Kandziora and Audige 17 , Reference Choo, Regev, Garfin and Kim 18 , Reference Jakubovic, Guha and Lu 32 As trainees and future faculty increase familiarity with CAN techniques and maximize their benefits, the cost-effectiveness of CAN, currently greatest in high-volume academic centers, may well trickle down to community institutions where a greater number of patients are treated.
One of the limitations of our nationwide survey is the 22.1% response rate. Although this of itself is not atypical for large national/multinational electronic surveys, non-responders in our cohort were predominantly in their PGY-1 or PGY-2 stage of training. This is likely because of a feeling among junior trainees that their operative experience to-date in their careers has been insufficient to comment adequately on the utility of navigation in their spine surgical training, confirmed anecdotally through conversations with the local residents at our institution. The utility of navigation for resident training may be felt perhaps even more so in the junior years; however, as the primary benefit, if applied correctly, is to confirm and enhance knowledge of spinal anatomy through real-time confirmation of visual and tactile feedback. Nonetheless, even among more senior trainees, we demonstrate a benefit in self-reported comfort with the use of navigation, a finding which may have been more robust with the inclusion of more junior trainees. Although our present investigation assesses only self-reported proficiency, future studies may assess the impact of intra-operative CAN on trainee proficiency with more objective metrics, such as quantitative screw accuracy and/or time required per screw.
Our retrospective database review is subject to the typical limitations of using an administrative database, including inconsistent coding particularly for pathology. Case complexity was not captured in the administrative database. Traumatic pathologies were heavily under-represented in this data set, at <5% of all cases. Data for the retrospective review encompasses a timeline of 2005–2014; significant changes in practice patterns may have occurred subsequent to this period, reflected anecdotally in both orthopedic surgery and neurosurgery at our local institution. Future studies with updated timelines are warranted.
Conclusions
In a large provincial cohort, intra-operative navigation was used for less than one-fifth of instrumented spinal fusions, more frequently by neurosurgeons than orthopedic spinal surgeons, and more often in academic than community institutions. At a trainee level, almost two-thirds of orthopedic surgical and neurosurgical trainees are not fully comfortable with the setup and use of CAN. The use of CAN improves self-reported trainee proficiency in placing thoracic instrumentation. Increasing practical education in spinal CAN from a trainee stage, particularly in orthopedic surgery and more so at the fellowship level, may increase adoption and maximize the benefits of CAN for the greatest population of patients.
Conflicts of Interest
VXDY is Chief Scientific Officer of 7D Surgical Inc., a surgical image-guidance company licensing an optical topographic imaging technology developed in the lab of VXDY. There are no financial or other conflicts of interest arising from this role. The remaining authors have no relevant conflicts of interest to disclose.
Financial Support
This research is supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canada Foundation for Innovation (CFI). Salary support for DG is provided in part by a Canadian Institutes of Health Research (CIHR) Postdoctoral Fellowship (FRN 142931).
Disclosures
AM, ZHJ, NMA, MGF, TGM, and AY have nothing to disclose.
DG reports grants from CIHR Postdoctoral Fellowship, during the conduct of the study.
VXDY reports non-financial support from 7D Surgical Inc., outside the submitted work.
Statement of Authorship
DG: study conception, study design, data collection, data analysis, drafting of manuscript, review of final manuscript.AM: data collection, data analysis, review of final manuscript. ZHJ: data collection, data analysis, drafting of manuscript, review of final manuscript. NMA: data analysis, review of final manuscript. MGF: study design, review of final manuscript, supervisory support. TGM: study design, review of final manuscript, supervisory support. AY: study design, review of final manuscript, supervisory support.VXDY: study design, review of final manuscript, supervisory support.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2017.376.








