INTRODUCTION
The 22q11.2 deletion syndrome (22q11DS), also known as velo-cardio-facial syndrome, is a genetic disorder caused by the deletion of a segment of chromosome 22 at the q11.2 band (Shapiro et al., Reference Shapiro, Tassone, Choudhary and Simon2014). 22q11DS affects approximately 1 out of every 1000–7000 births (Botto et al., Reference Botto, May, Fernhoff, Correa, Coleman, Rasmussen and Campbell2003; Grati et al., Reference Grati, Molina Gomes, Ferreira, Dupont, Alesi, Gouas and Vialard2015) and is characterized by immunologic deficiencies, hypocalcemia, congenital cardiac anomalies, palate anomalies, and mild facial dysmorphism (Shprintzen, Reference Shprintzen2000).
Psychotic symptoms are present in nearly 30% of the 22q11DS adult population, and about 22% later develop schizophrenia or schizoaffective disorder (Bassett et al., Reference Bassett, Chow, Husted, Weksberg, Caluseriu, Webb and Gatzoulis2005). In addition to psychosis, attention deficit hyperactivity disorder (ADHD) (15–37% prevalence rates depending upon age) and anxiety disorders (27–35%) are especially prevalent in individuals with 22q11DS (Schneider et al., Reference Schneider, Debbane, Bassett, Chow, Fung, van den Bree and Eliez2014). Individuals with 22q11DS also have social adjustment problems and lower abilities across multiple domains of cognition (Fung et al., Reference Fung, Butcher, Costain, Andrade, Boot, Chow and Bassett2015). Intellectual abilities of individuals with 22q11DS are impaired when compared to the typically developing population, with mean Full Scale Intelligence Quotients (FSIQ) in the borderline range of intelligence (Vorstman et al., Reference Vorstman, Breetvelt, Duijff, Eliez, Schneider, Jalbrzikowski and Bassett2015).
Executive Functioning in 22q11DS
In addition to generalized intellectual deficits, individuals with 22q11DS have executive functioning (EF) impairments (Antshel et al., Reference Antshel, Fremont and Kates2008). EF is an umbrella term that encompasses various cognitive domains, including cognitive flexibility, planning, self-regulation, and working memory (Anderson, Reference Anderson2002; Harms et al., Reference Harms, Zayas, Meltzoff and Carlson2014). EF abilities develop in a progressive fashion, emerging in early childhood and developing into adulthood (Bunge & Zelazo, Reference Bunge and Zelazo2006; Maeder et al., Reference Maeder, Schneider, Bostelmann, Debbané, Glaser, Menghetti and Eliez2016). Childhood EF abilities predict adolescent and adult academic and occupational functioning in individuals with and without ADHD (Miller et al., Reference Miller, Nevado-Montenegro and Hinshaw2012).
Inhibition (Shapiro et al., Reference Shapiro, Tassone, Choudhary and Simon2014), working memory (Maeder et al., Reference Maeder, Schneider, Bostelmann, Debbané, Glaser, Menghetti and Eliez2016), and cognitive flexibility (Antshel et al., Reference Antshel, Shprintzen, Fremont, Higgins, Faraone and Kates2010) deficits have been reported in the 22q11DS population. Most of the literature reports lower performance in all EF domains for individuals with 22q11DS when compared to the typically developing population (Antshel et al., Reference Antshel, Fremont and Kates2008). Nonetheless, there are mixed findings about the developmental trajectory of EF in individuals with 22q11DS, and whether EF improves as a function of age and time. Shapiro et al. (Reference Shapiro, Tassone, Choudhary and Simon2014) reported that working memory develops in the same trajectory as in the typically developing population, just with weaker performance across time compared with age-norms (i.e., standardized scores). Others (Chawner et al., Reference Chawner, Doherty, Moss, Niarchou, Walters, Owen and van den Bree2017) have similarly reported that EF improves over time yet never reaches the levels of typically developing peers. Conversely, Maeder et al. (Reference Maeder, Schneider, Bostelmann, Debbané, Glaser, Menghetti and Eliez2016) found that individuals with 22q11DS do not improve in EF with age and time when compared with age-norms (i.e., standardized scores) in two domains of EF: verbal fluency and auditory/verbal working memory. Antshel and colleagues followed children with 22q11DS into adulthood and reported that after controlling for FSIQ differences, children with 22q11DS differed significantly from controls in longitudinal trajectories of visual and auditory/verbal working memory yet not in other EF trajectories. In both working memory domains, differences between controls and individuals with 22q11DS increased over time (Antshel et al., Reference Antshel, Fremont, Ramanathan and Kates2017).
Executive functions and psychosis
Cross-sectionally, EF deficits have been reported to have inconsistent associations with psychosis in children with 22q11DS (Lajiness-O’Neill et al., Reference Lajiness-O’Neill, Beaulieu, Asamoah, Titus, Bawle, Ahmad and Pollack2006; Niarchou et al., Reference Niarchou, Zammit, van Goozen, Thapar, Tierling, Owen and van den Bree2014). In adolescence and young adulthood, EF deficits have also been inconsistently associated with psychotic symptoms; some findings show positive associations (Chow et al., Reference Chow, Watson, Young and Bassett2006; van Amelsvoort et al., Reference van Amelsvoort, Henry, Morris, Owen, Linszen, Murphy and Murphy2004; Weinberger et al., Reference Weinberger, Yi, Calkins, Guri, McDonald-McGinn, Emanuel and Gothelf2016), yet one study demonstrated no association (Yi et al., Reference Yi, Calkins, Tang, Kohler, McDonald-McGinn, Zackai and Gur2015). In addition to the inconsistent nature of these findings, cross-sectional studies do not permit investigating the temporal nature of the relationship between EF and psychosis.
Longitudinally, one study reported that childhood EF predicted psychotic symptoms in mid-adolescence (Antshel et al., Reference Antshel, Shprintzen, Fremont, Higgins, Faraone and Kates2010) yet this study is limited by the short follow-up period of 3 years. In a longer follow-up period of 9 years, in the same cohort, Antshel and colleagues reported that cognitive flexibility trajectories predicted psychotic symptoms in adulthood. Individuals with 22q11DS who developed psychotic symptoms improved less appreciably and continued to demonstrate difficulties with cognitive flexibility relative to individuals with 22q11DS who did not have psychotic symptoms (Antshel et al., Reference Antshel, Fremont, Ramanathan and Kates2017). To our knowledge, no other study has considered the association between childhood EF and psychosis using a longitudinal design. Given the consistent associations between childhood EF and adult psychosis noted in the idiopathic psychosis literature (Fusar-Poli et al., Reference Fusar-Poli, Borgwardt, Bechdolf, Addington, Riecher-Rossler, Schultze-Lutter and Yung2013), this represents a void in the 22q11DS literature.
Executive functions and psychosocial functional outcomes
Cross-sectionally, mixed results have been reported about the associations between EF abilities and psychosocial outcomes. For example, in adolescents with 22q11DS, IQ was associated with social competence abilities, while EF was not (Campbell et al., Reference Campbell, McCabe, Melville, Strutt and Schall2015). In children with 22q11DS, however, a cross-sectional association between social functioning and EF has been reported (Kiley-Brabeck & Sobin, Reference Kiley-Brabeck and Sobin2006). Longitudinally, while there have been multiple studies investigating which childhood intellectual variables best predict psychosocial 22q11DS outcomes in adolescence and adulthood (Fiksinski et al., Reference Fiksinski, Breetvelt, Duijff, Bassett, Kahn and Vorstman2017; Gothelf et al., Reference Gothelf, Schneider, Green, Debbane, Frisch, Glaser and Eliez2013; Hooper et al., Reference Hooper, Curtiss, Schoch, Keshavan, Allen and Shashi2013; Schneider et al., Reference Schneider, Debbane, Bassett, Chow, Fung, van den Bree and Eliez2014; Tang et al., Reference Tang, Moore, Calkins, Yi, McDonald-McGinn, Zackai and Gur2017), surprisingly none of these studies have investigated EF – all focused on IQ. Given the predictive abilities of childhood EF to later social (Rinsky & Hinshaw, Reference Rinsky and Hinshaw2011), educational (Sjowall et al., Reference Sjowall, Bohlin, Rydell and Thorell2017), and occupational (Barkley & Fischer, Reference Barkley and Fischer2011) functioning outcomes in the non-22q11DS literatures, this also represents a void in the 22q11DS literature.
Rationale for Current Study
While multiple studies have reported EF deficits in 22q11DS across the lifespan, very few studies have investigated the relationship between childhood EF and adult outcomes. The present study aims to fill this research void. While one previous study (Antshel et al., Reference Antshel, Fremont, Ramanathan and Kates2017) investigated longitudinal trajectories of EF as predictors for psychosis, the study (1) did not investigate childhood EFs per se as predictors, (2) did not consider parent report of EF as a variable and (3) only focused on psychosis. Thus, we presently know very little about how childhood EF is associated with adult outcomes. In addition to being innovative, this research question also holds potential clinical significance. Findings from the present study may permit a greater understanding of which children with 22q11DS are at greatest risk for negative developmental outcomes, and may facilitate early, targeted intervention and prevention strategies.
Given the non-22q11DS research findings, we hypothesize that childhood EF will be negatively associated with adult functioning whereas verbal learning and memory (a non-EF ability) will not. More specifically, we hypothesized that childhood EF abilities would be less well developed in children with 22q11DS compared to control participants. Trajectories of EF abilities would be similar between 22q11DS and control participants. Finally, we hypothesized that study group would not have a moderating effect on the relationship between child EF and young adult outcomes.
METHODS
Participants
This 9-year longitudinal study followed individuals with 22q11DS across four time points, with data collected at baseline, year 3, year 6, and year 9. Children with 22q11DS were initially recruited from a large academic medical center in the northeastern United States during the years 2002–2005. Only those children with a fluorescence in situ hybridization-confirmed deletion of 22q11.2 were included. All families who met inclusion criteria agreed to participate. Specifically, children with 22q11DS, and their age and gender matched siblings were recruited from the Center for the Diagnosis, Treatment and Study of Velo-Cardio-Facial Syndrome at SUNY-Upstate Medical University. In addition, age and gender matched community control participants were recruited from local public schools via advertisements. Children in the community control group were excluded if they were not instructed in a general education classroom. In all three groups, children with an identifiable genetic disorder other than 22q11DS or children with an identifiable neurological condition (e.g., traumatic brain injury, pre-term birth) that is known to affect cognitive or psychiatric function were excluded from participation. Given the developmental delays that are associated with 22q11DS, no attempt was made to exclude sibling or community control participants with ADHD and learning disabilities. Due to the lack of significant differences on all independent and dependent variables between our sibling controls and community controls (p’s>.60), the two control groups were combined to form one control group. Our control group consisted of 22 community controls and 21 sibling controls.
For the current project, only participants who had Time 4 (young adult) data and who also had baseline data were included. Our total sample consisted of 63 children with 22q11DS (34 males) and 43 control participants (22 males). No gender differences, X2 (1)=1.22, p=.543 or differences in age between the two groups existed at both baseline, F (1104)=1.98, p=.161: 22q11DS=12.2 years (SD=2.3); Controls=11.8 (SD=2.0), and young adulthood, F (1104)=0.63, p=.424: 22q11DS=21.2 years (SD=2.2); Controls=20.9 (SD=2.0). No Hollingshead scale socioeconomic status or racial/ethnicity differences were present between the two groups (p>.05).
In young adulthood, control participants were more likely to live independently (27%) compared to young adults with 22q11DS (10%), X2 (1)=5.10, p=.024. Likewise, control participants were more likely to be employed (77%) compared to young adults with 22q11DS (46%), X2 (1)=9.48, p=.002. Within the subsample of those who were employed, control participants worked more hours per week (30.38 hours per week, SD=9.98) compared with young adults with 22q11DS (14.69 hours per week, SD=9.51), F (1, 62)=37.54, p<.001, η2=.40.
An independent samples t-test found no differences in attrition between our two groups, t (1)=3.44, p=.222. Furthermore, participants lost to follow-up did not differ from those retained on any relevant Time 1 sociodemographic measures including participant age, gender, and socioeconomic status. In addition, participants lost to follow-up did not differ from those retained on any relevant Time 1 functional or cognitive variables (p>.05). Thus, those participants who completed Time 4 assessments appear representative of the Time 1 sample.
Measures
Young adult intellectual ability measure
Time 4 Wechsler Adult Intelligence Scale – third edition
The Wechsler Adult Intelligence Scale – Third edition (WAIS-III; Wechsler, Reference Wechsler1993) is a commonly used psychological test for measuring intellectual abilities. For the current study, only the FSIQ was used. Please see Supplemental Table 1 for a comprehensive table detailing each measure used in the current study.
Young adult parent report dependent variables
Time 4 Vineland Adaptive Behavior Scales – second edition
The Vineland Adaptive Behavior Scales – second edition (VABS-II; Sparrow et al., Reference Sparrow, Cicchetti and Balla2005) Parent/Caregiver Rating Form asks parents to rate their adult child’s ability to independently perform behaviors across three domains: Communication, Daily Living Skills, and Socialization. Standard scores (M=100, SD=15) are provided for each domain, with higher scores indicating better functioning. For the current study, only the VABS-II Total Composite was used.
Time 4 Behavior Rating Inventory of Executive Functioning – adult version
The Behavior Rating Inventory of Executive Functioning – adult version (BRIEF-A; Roth et al., Reference Roth, Isquith and Gioia2006) is an ecologically standardized measure that assesses a collateral reporter’s views of an adult’s EF in their everyday environment. The BRIEF-A contains 75 items that load onto 9 subdomains. The Global Executive Composite (GEC) Index, which represents the overall EF score, was used in this study. Raw scores are transformed into T scores for results interpretation, with higher scores indicate weaker EF abilities.
Young adult clinician rated dependent variable
Time 4 Structured Interview for Prodromal Symptoms
The Structured Interview for Prodromal Symptoms (SIPS; Miller et al., Reference Miller, McGlashan, Rosen, Cadenhead, Cannon, Ventura and Woods2003) is a commonly used structured interview that evaluates current psychosis symptoms and clinical risk of psychosis. Previous research indicates that the SIPS has good predictive value of correctly identifying 67% of individuals who later developed psychosis at a 24-month follow-up (Miller et al., Reference Miller, McGlashan, Rosen, Cadenhead, Cannon, Ventura and Woods2003). In the current study, the full SIPS was administered both to participants and to their parents in young adulthood (Time 4). If a discrepancy existed between raters, the more severe rating was entered by the clinician.
For the current study, only the Positive Symptom domain score was used in analyses. This decision was based upon positive symptoms being more specific to psychosis than negative symptoms (Keshavan et al., Reference Keshavan, Nasrallah and Tandon2011), especially in a condition such as 22q11DS, in which the majority of individuals have negative symptoms such as social isolation (Schneider et al., Reference Schneider, Debbane, Bassett, Chow, Fung, van den Bree and Eliez2014). The Positive Symptom domain includes questions related to the presence of positive psychotic symptoms, such as unusual thought content, suspiciousness, ideas of grandiosity or persecution with delusional features, hallucinations, and disorganized speech. Higher scores on the SIPS indicate the presence of more positive symptoms of psychosis. No control participants were reported to have psychotic symptoms. In contrast, 22% of participants with 22q11DS were reported to have psychotic symptoms at Time 4 (p<.001).
Young adult self-report dependent variable measures
Time 4 ecologically valid functioning measures
Information regarding the young adult’s living status and occupation was obtained. Living status was dichotomized into independent (living on his/her own) or dependent (living with parents, living in group home, etc.). Employment was dichotomized into employed/nonemployed. For those who were employed, a continuous measure of the number of hours per week was obtained.
Time 4 Behavior Rating Inventory of Executive Functioning – adult version
The Behavior Rating Inventory of Executive Functioning – adult version (BRIEF-A; Roth et al., Reference Roth, Isquith and Gioia2006) was used to assess self-report of EF in his/her everyday environment. Please see previous description of the BRIEF-A.
Time 4 Adult Self-Report
The Adult Self Report (ASR; Achenbach & Rescorla, Reference Achenbach and Rescorla2003) scale was used to assess self-perceptions of symptoms in the young adults with 22q11DS. The ASR consists of 123 statements that load onto 8 scales. The ASR has solid psychometric properties (Achenbach & Rescorla, Reference Achenbach and Rescorla2003). For the current study, only the ADHD Problems, Internalizing and Externalizing composites were used. This decision was made due to the high prevalence of psychopathology, especially ADHD, in 22q11DS (Schneider et al., Reference Schneider, Debbane, Bassett, Chow, Fung, van den Bree and Eliez2014), the associations between ADHD and executive dysfunction (Hervey et al., Reference Hervey, Epstein and Curry2004) as well as a desire to reduce the likelihood of a Type I error.
Time 4 Social Adjustment Scale – Self-Report
The Social Adjustment Scale - Self-Report (SAS-SR; Weissman, Reference Weissman1999) is a 54-item self-report scale that measures social adjustment. The measure is intended for individuals ages 17 years and older and identifies six social role areas: work, social and leisure activities, relationships with extended family, role as a spouse or partner, parental role, and role within the family unit. An area is not assessed if the respondent indicates that the questions are not relevant to him or her (i.e., if the respondent does not have children or is not married). Each item is rated on a five-point Likert scale (1=always, 5=never). Scores are transformed into T-scores (M=50, SD=10) based on a normative sample, with higher scores indicating more social impairment. For the current study, only the SAS-SR Total Composite was used.
Time 4 Trait Emotional Intelligence Questionnaire – Short Form
The Trait Emotional Intelligence Questionnaire – Short Form (TEIQue-SF; Cooper & Petrides, Reference Cooper and Petrides2010) is a 30-item questionnaire based on the full, 153–item TEIQue (Petrides, Reference Petrides2009), which is designed to measure 15 facets of emotional intelligence, including traits of adaptability, assertiveness, emotion expression, emotion perception, emotion regulation, emotion management, impulse control, relationships, self-esteem, self-motivation, social awareness, stress management, trait empathy, trait happiness, and trait optimism. Two items from each of these facets of emotional intelligence were included in the Short Form, based on their correlation with total facet scores from the full form. A single, global score for Emotional Intelligence is derived and was used in the current study. The Short Form has solid psychometric properties (Cooper & Petrides, Reference Cooper and Petrides2010).
Childhood executive functioning predictors
Wechsler Intelligence Scale for Children – 3rd edition
The Wechsler Intelligence Scale for Children – 3rd edition (WISC-III; Wechsler, Reference Wechsler1991) Digit Span was used to assess working memory.
Gordon Diagnostic System
The Gordon Diagnostic System (GDS; Gordon, Reference Gordon1983) is a performance-based continuous performance test that measures sustained attention and response inhibition. For the present study, only the standardized omission and commission errors scores were used in the analyses.
Tower of London
The Tower of London (TOL; Shallice, 1982) has demonstrated good construct validity as a measure of planning (Culbertson & Zillmer, Reference Culbertson and Zillmer1998). The total number of moves was used in analyses.
Wisconsin Card Sorting Test
The Wisconsin Card Sorting Test (WCST; Heaton et al., Reference Heaton, Chelune, Talley, Kay and Curtiss1993) is a task that measures cognitive flexibility. Standard scores for perseverative errors and non-perseverative errors were used in our analyses. The WCST was manually administered.
Stroop Color-Word Test
The Stroop Color-Word Test (Golden, Reference Golden1978) measures cognitive flexibility, selective attention, and response inhibition. The interference T-score was used in the present study.
Visual Span Test
The Visual Span Test (VSPAN; Davis, 1998) is a computer-based test that assesses visual working memory abilities. The forward and backward span standardized z-scores were used in the current study.
Behavior Rating Inventory of Executive Functioning
The Behavior Rating Inventory of Executive Functioning (BRIEF; Gioia et al., Reference Gioia, Isquith, Guy and Kenworthy2000) is an ecologically standardized measure that assesses a parent’s views of a child’s EF in his or her everyday environment. For the current study, only the two composites, the Behavioral Regulation Index and the Metacognitive Index, were used.
Childhood nonexecutive functioning predictor
To assess the specificity of these findings to EF, a learning/memory measure was used as a control variable.
California Verbal Learning Test – children’s version
The California Verbal Learning Test-Children’s Version (CVLT-C; Delis et al., Reference Delis, Kramer, Kaplan and Ober1994) measures auditory/verbal learning and working memory. Standardized scores for List A Trial 1 (recall after hearing the list once), List A Trial 5 (recall after hearing the list five times), List B (interference), Short Delay Recall, Long Delay Recall, and Perseverations/Intrusions were used in analyses.
Procedures
Informed consent and assent were attained from parents and participants, and all data included in this manuscript were obtained in compliance with regulations of SUNY-Upstate Medical University. At both time periods, a doctoral-level examiner administered all psychological tests to participants in a quiet room. Parents completed all parent-report rating scales in a separate room.
Statistical Analyses
All analyses were conducted in SPSS Statistics Version 23, with statistical significance set to p<.05. When appropriate, effect size is reported using eta squared (η2) or Cohen’s d. First, t-tests were used to examine group mean differences between children with 22q11DS and control children on baseline EF abilities and adaptive behavior. Repeated measures analysis of variance (ANOVA) were then used to examine parent reported EF trajectories in children with 22q11DS and control children from baseline to young adulthood.
Due to the multivariate and intercorrelated nature of the EF data collected from participants at Time 1, principal components analysis (PCA) was used as a data reduction technique to identify the key components of EF. Twelve different EF variables, including both rater-based and performance-based measures, were included in the PCAs, as listed in Table 2. In addition, CVLT-C variables were included in the PCA to test the specificity of our findings to EF. PCA on these 18 items was conducted using Varimax rotation and Kaiser normalization. All components with Eigen values greater than 1 were considered to be meaningful factors. A loading cutoff of 0.4 was used to determine which EF measures loaded onto each identified factor. PCA was conducted across the total sample.
Before proceeding with analyses examining childhood EF as a predictor of young adult outcomes in individuals with 22q11DS, all young adult outcome variables of interest were examined for normality. For normally distributed outcome variables, stepwise linear regression was used, with the first step including the relevant baseline (childhood) dependent variable as a covariate, and the second step including the EF factors identified through the PCA.
For non-normally distributed outcome variables, negative binomial regression was used. Similar to the linear regression analyses, the first step in the negative binomial regressions included the relevant baseline (childhood) dependent variable. Models that were trending toward significance were re-examined using only factors identified as being significant predictors of previously examined outcomes, to increase statistical power. For each outcome variable that was significantly predicted by childhood EF among individuals with 22q11DS, models were re-examined, adding baseline IQ as a first step, in order to determine whether EF uniquely predicts these outcomes, or whether IQ might be driving these relationships.
As a final step, for each outcome variable that was significantly predicted by childhood EF among individuals with 22q11DS, models were re-examined including control participants. Only the EF factors that were significant predictors for individuals with 22q11DS were included in these models, and study group and interaction terms between study group and each EF factor were added to determine whether the study group has a moderating effect on the relationship between EF and young adult outcomes.
RESULTS
Executive Functioning in 22q and Control Children
Childhood variables
Consistent with our hypothesis, children with 22q11DS performed worse on all childhood EF measures compared to control children (all p’s ≤ .01), except for Stroop interference, GDS total, and digit span forward scores. Effect sizes ranged from medium to large (Cohens d=0.5–1.9). Children with 22q11DS also had significantly more impaired functioning in each domain of adaptive behavior measured by the Vineland at Time 1 (all p’s<.001). Effect sizes were all large (Cohens d=1.2–1.6). See Table 1 for a summary of means and standard deviations for participants with and without 22q11DS on baseline measures.
Table 1 Baseline cognitive functioning and adaptive functioning
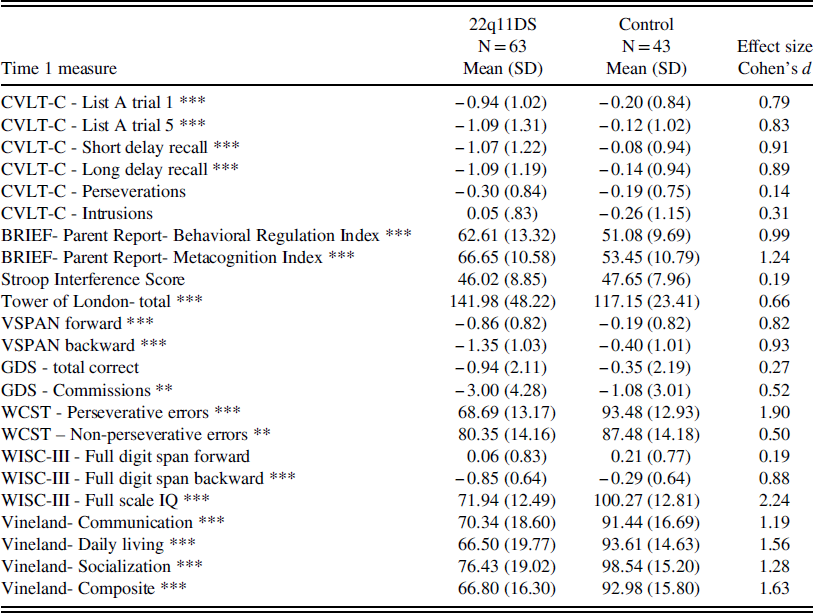
Note: BRIEF=Behavioral Rating Inventory of Executive Functioning; WISC-III=Wechsler Intelligence Scale for Children- 3rd edition; WCST=Wisconsin Card Sorting Test; Stroop=Stroop Color-Word Test; CVLT-C=California Verbal Learning Test-Children’s version. VSPAN=Visual Span. GDS=Gordon Diagnostic System Continuous Performance Test.
*p<.05; **p<.01; ***p<.001.
Parent reported EF developmental trajectory
A 2x2 repeated measures ANOVA examining the effects of time (from T1 to T4) and diagnostic status (22q vs. control) on the BRIEF-A GEC revealed main effects of both time (F(1, 50)=18.87, p<.001, η2=.27) and diagnosis (F(1, 50)=48.91, p<.001, η2=.49), such that parent-reported EF problems declined slightly from time 1 to time 4 in both groups, and EF was more impaired in 22q11DS than controls at both times. Consistent with our hypothesis, there was no significant interaction between group and time (F(1, 50)=.16, p=.69), suggesting that participants with 22q11DS begin with and maintain significantly greater parent- reported impairments in EF compared to control participants.
Principal Components Analysis
PCA using Varimax rotation and Kaiser normalization was conducted on the entire sample with all Time 1 EF and verbal learning measures and resulted in a six-factor solution. Factor 1 explained 31% of the variance (Eigen value=5.64), consisted of all CVLT-C measures (trial 1, trial 5, short delay performance, long delay performance, intrusions, and perseverations) and was determined to represent an auditory verbal learning and memory factor. Higher scores on this factor indicate better verbal learning and memory. Factor 2 explained 10% of the variance (Eigen value=1.76), consisted of WISC-III digit span backward, WCST perseverative and non-perseverative errors, and TOL measures and was determined to represent a global EF factor. Higher scores on this factor indicate better global EF. Factor 3 explained 9% of the variance (Eigen value=1.64), consisted of GDS commissions, GDS total correct, CVLT-C intrusions, and TOL measures and was determined to represent a response inhibition factor. Higher scores on this factor indicate better response inhibition. Factor 4 explained 7% of the variance (Eigen value=1.20), consisted of VSPAN forward, VSPAN backward, and TOL and was determined to represent a visual working memory factor. Higher scores on this factor indicate better visual working memory. Factor 5 explained 6% of the variance (Eigen value=1.10), consisted of BRIEF metacognition and behavioral regulation indices and was determined to represent a parent report of child EF factor. Lower scores on this factor indicate better parent-reported EF. Factor 6 explained 6% of the variance (Eigen value=1.05), consisted of Stroop Interference and WISC-III digit span forward, and was determined to represent a self-monitoring factor. Higher scores on this factor indicate better self-monitoring. See Table 2 for the rotated component matrix. For interpretation of the following results, it is important to note that for factor 5, lower scores are better, whereas for all other PCA factors, higher scores are better.
Table 2 Rotated component matrix from principal components analysis of time 1 executive functioning measures
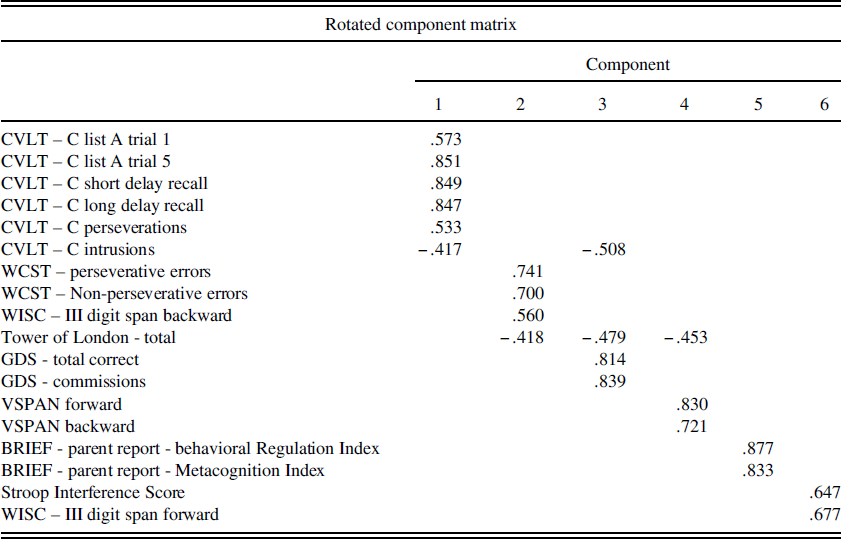
Note: BRIEF=Behavioral Rating Inventory of Executive Functioning; WISC-III=Wechsler Intelligence Scale for Children- 3rd edition; WCST=Wisconsin Card Sorting Test; Stroop=Stroop Color-Word Test; CVLT-C=California Verbal Learning Test-Children’s version. VSPAN=Visual Span. GDS=Gordon Diagnostic System Continuous Performance Test.
Childhood EF as a Predictor of Young Adult Outcomes
Using the six factors identified through the PCA, childhood EF was examined as a predictor of young adult outcomes.
Predicting young adult psychopathology
Negative binomial regression revealed that for individuals with 22q11DS, childhood EF significantly predicted SIPS clinician rated positive symptoms of psychosis in young adulthood, with factors 5 (parent report of childhood EF; B=2.02, p<.05) and 6 (self-monitoring; B=−3.41, p<.01) reaching significance. Across all factors, and consistent with our hypothesis, worse childhood EF skills were predictive of greater SIPS symptoms of psychosis in young adulthood. Factors 5 and 6 remained significant when baseline IQ was added as a covariate in the model, suggesting that childhood EF is a unique predictor of young adult psychotic symptoms.
Stepwise linear regression revealed no significant effects of childhood EF on ASR young adult self-reported internalizing problems among individuals with 22q11DS. However, there were significant effects of childhood EF on ASR young adult self-reported externalizing problems. Factors 2 (global EF; B=−7.42, p<.05) and 5 (parent report of childhood EF; B=12.61, p<.001) significantly predicted ASR self-reported externalizing problems in young adults with 22q11DS. When baseline IQ was added as a covariate in the model, factor 5 remained significant, suggesting that EF is a unique predictor of externalizing problems. Factor 2 was no longer significant; however, this is likely because factor 2 includes digit span, which is a subcomponent of IQ. Inconsistent with our hypothesis, when control participants were included in the model predicting externalizing problems, and the study group was examined as a potential moderator with factors 2 and 5, there was a significant study group x factor 2 interaction (B=−8.30, p<.01). This suggests that the relationship between global EF and young adult externalizing problems is different for individuals with and without 22q11DS. More specifically, worse childhood global EF is associated with greater young adult externalizing problems for individuals with 22q11DS, but not for control participants. Please see Supplemental Tables 2–7 for regression analyses including IQ as a covariate.
There was a trend toward significance when predicting self-reported ASR young adult ADHD problems from childhood EF among individuals with 22q11DS (F=2.233, p=.078). When only factors 2, 5, and 6 were included in the model, the model became significant (F=3.75, p<.05), with factors 2 (global EF; B=−4.55, p<.01) and 5 (parent report of childhood EF; B=6.45, p<.01) significantly predicting ASR young adult ADHD problems. When baseline IQ was added as a covariate in the model, factor 5 remained significant, suggesting that EF is a unique predictor of ADHD problems. Factor 2 was no longer significant; however, this is likely because factor 2 includes digit span, which is a subcomponent of IQ. Also inconsistent with our hypothesis, when control participants were included in the model predicting ADHD problems, and the study group was examined as a potential moderator with factors 2 and 5, there was a significant study group x factor 2 interaction (B=−8.79, p<.01). This suggests that the relationship between global EF and young adult ADHD problems is different for individuals with and without 22q11DS. More specifically, worse childhood global EF is associated with greater young adult ADHD problems for individuals with 22q11DS, but not for individuals without 22q11DS.
Predicting young adult psychosocial functioning
For young adult social, emotional, and intellectual functioning outcomes, stepwise linear regression revealed that childhood EF significantly predicted VABS-II parent-reported young adult adaptive behavior among individuals with 22q11DS, even after accounting for childhood adaptive behavior. More specifically, Factor 5 (parent report of childhood EF) was a significant predictor of VABS-II parent-reported young adult adaptive behavior (B=7.79, p<.01). When baseline IQ was added as a covariate in the model, factor 5 remained significant, suggesting that parent report of childhood EF is a unique predictor of young adult adaptive behavior. When control participants were included in the model predicting adaptive functioning, and study group was examined as a potential moderator with factor 5, there was not a significant interaction (p>.05). Consistent with our hypothesis, this suggests that childhood EF predicts adaptive functioning similarly for individuals with and without 22q11DS.
Stepwise linear regression revealed a trend toward significance when predicting SAS-SR young adult self-reported social adjustment among individuals with 22q11DS (F=1.884, p=.132). When only factors 2, 5, and 6 were included in the model, the model was significant (F=2.884, p<.05), with factor 5 (parent report of child EF; B=9.40, p<.01) significantly predicting social adjustment, after covarying for childhood adaptive behavior. When baseline IQ was added as a covariate in the model, factor 5 remained significant, suggesting that EF is a unique predictor of SAS-SR young adult self-reported social adjustment. When control participants were included in the model predicting social adjustment, and the study group was examined as a moderator with factor 5, there was not a significant interaction (p>.05). Consistent with our hypothesis, this suggests that childhood EF predicts young adult social adjustment similarly for individuals with and without 22q11DS.
Childhood EF also significantly predicted TEIQue-SF young adult emotional intelligence abilities among individuals with 22q11DS, even when accounting for childhood adaptive behavior. More specifically, factors 4 (visual working memory; B=.574, p<.01) and 5 (parent report of child EF; B=-.399, p<.05) were significant predictors of TEIQue-SF emotional intelligence. When baseline IQ was added as a covariate in the model, factor 5 remained significant, suggesting that parent report of childhood EF is a unique predictor of emotional intelligence. Factor 4 was no longer significant, possibly because working memory is a subcomponent of IQ. When control participants were included in the model predicting emotional intelligence, and the study group was examined as a moderator with factors 4 and 5, there was a significant study group x factor 4 interaction (B=.512, p<.05). Inconsistent with our hypothesis, this suggests that the relationship between visual working memory and young adult emotional intelligence is different for individuals with and without 22q11DS. More specifically, worse childhood visual working memory is associated with lower TEIQue-SF emotional intelligence for individuals with 22q11DS, but not for individuals without 22q11DS. See Figure 1 for a visual depiction of this interaction.
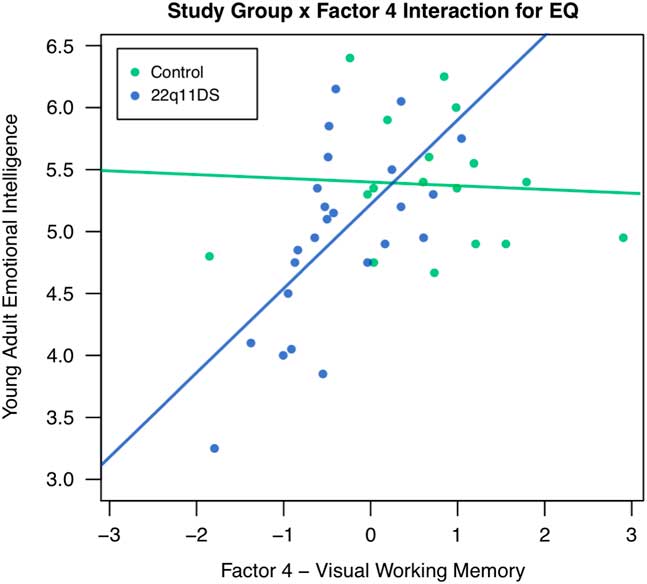
Fig. 1 Study group as a moderator of the relationship between factor 4 – Visual Working Memory and Young Adult Emotional Intelligence. Note: EQ=Emotional Intelligence.
Finally, with regard to employment and independent living status, no childhood EF variables significantly predicted young adult status. Likewise, for those who were employed in young adulthood, no childhood EF variables predicted number of hours worked per week. See Table 3 for a summary of the significant regression analyses predicting young adult outcomes for individuals with 22q11DS.
Table 3 Statistically significant young adult outcomes from childhood executive functioning in individuals with 22q11DS
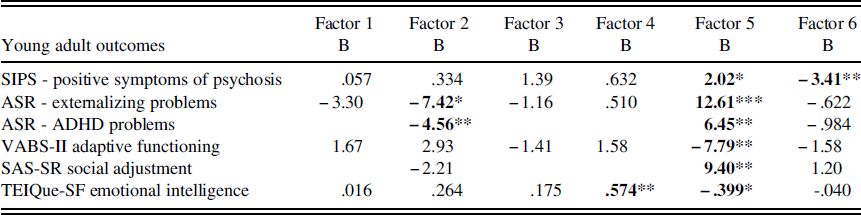
Note: SIPS=Structured Interview for Prodromal Symptoms; ASR=Adult Self-Report scale. VABS-II=Vineland Adaptive Behavior Scales- 2nd edition; SAS-SR=Social Adjustment Scale – Self-report; TEIQue-SF=Trait Emotional Intelligence Questionnaire – Short Form.
*p<.05; **p<.01; ***p<.001.
DISCUSSION
Consistent with previous literature, children with 22q11DS displayed significantly impaired EF compared to control children (Shapiro et al., Reference Shapiro, Tassone, Choudhary and Simon2014). A more innovative finding, however, is that childhood EF was predictive of several key outcome variables in young adults with 22q11DS, including parent report of adaptive behavior, positive symptoms of psychosis, emotional intelligence, and self-reported social adjustment and externalizing problems. While previous literature suggests that 22q11DS is associated with the functional impairments and psychopathology examined in the present study (Maeder et al., Reference Maeder, Schneider, Bostelmann, Debbané, Glaser, Menghetti and Eliez2016), our study is the first to examine whether childhood EF predicts young adult functioning in individuals with 22q11DS. The findings of the present study suggest that EF may be a worthwhile target for intervention among children with 22q11DS, in order to reduce the likelihood that these children will go on to develop negative outcomes in young adulthood. While some authors (Cutler-Landsman, Reference Cutler-Landsman2012) have previously recommended that parents and educators of children with 22q11DS target EF development due to concurrent difficulties, our data are the first to indicate that childhood EF abilities also have clear distal effects well into adulthood.
Across the six factors identified in this study, only EF factors were significant predictors. Parent report of child EF was the most consistent predictor of young adult outcomes and was a stronger predictor than the psychological tests of EF. This may be explained because parents are reporting on their observations of their child’s EF in real-life situations, whereas neuropsychological tests of EF examine children’s EF in a more controlled environment, which may have less ecological validity. This is consistent with findings from Barkley and Fischer (Reference Barkley and Fischer2011), who reported that EF ratings are better predictors of adult outcomes than EF tests, because these tests may not be capturing the same complexity of EF required in real-world situations.
Our findings suggest that EF is a unique predictor of young adult outcomes for individuals with 22q11DS. The auditory verbal learning factor (factor 1) did not significantly predict any of the outcomes examined, while four out of five EF factors were significant predictors of at least one outcome. Additionally, parent-reported EF continued to be a significant predictor even after IQ was included as a covariate in each model, suggesting that the predictive power of EF cannot be explained simply by differences in IQ. In light of the controversy inherent in the neuropsychology field about covarying for IQ in neurodevelopmental disorder populations (Dennis et al., Reference Dennis, Francis, Cirino, Schachar, Barnes and Fletcher2009), these data provide clear guidance to clinicians: Childhood EF abilities are a relevant factor to consider, over and above IQ.
The study group moderated the relationship between childhood EF and young adult outcomes for some outcomes but not for others. Study group did not moderate the relationship between childhood EF and young adult adaptive behavior or social adjustment. This suggests that EF predicts adaptive behavior and social adjustment similarly for individuals with and without 22q11DS. However, the study group moderated the relationship between childhood EF and emotional intelligence, externalizing problems, and ADHD problems. For each of these outcomes, lower childhood EF scores were associated with poorer young adult outcomes for individuals with 22q11DS, but not for individuals without 22q11DS. Of note, there were no interactions of study group with factor 5 (parent-report of childhood EF), suggesting that parent-reported EF predicts outcomes similarly across individuals with and without 22q11DS.
Our finding that childhood deficits in EF predict positive prodromal symptoms in adulthood are generally consistent with several longitudinal studies that have reported deficits in cognitive functioning in children who eventually develop idiopathic schizophrenia (Cannon et al., Reference Cannon, Bearden, Hollister, Rosso, Sanchez and Hadley2000; Dickson et al., Reference Dickson, Laurens, Cullen and Hodgins2012; Fuller et al., Reference Fuller, Nopoulos, Arndt, O’Leary, Ho and Andreasen2002; Seidman et al., Reference Seidman, Buka, Goldstein and Tsuang2006; Woodberry et al., Reference Woodberry, Giuliano and Seidman2008). Although many of these studies relied on global IQ scores rather than individual neuropsychological tests, specific developmental delays in the cognitive skills involved in working memory have been observed (Reichenberg, Reference Reichenberg2010). Although we are not aware of longitudinal studies that specifically predict emotional intelligence in adulthood, deficits in emotional intelligence have been observed in both individuals with 22q11DS and idiopathic schizophrenia, attesting to the importance of identifying early predictors of deficits in this skill set (Ho et al., Reference Ho, Radoeva, Jalbrzikowski, Chow, Hopkins, Tran and Bearden2012; Kee et al., Reference Kee, Horan, Salovey, Kern, Sergi, Fiske and Green2009).
Given the predictive utility of EF, implementing receiver operating characteristic curves to detect cut-off scores for low EF performance and risk for poor outcomes could improve detection of high risk children. Future analyses could also integrate the results of these analyses with other variables that may best predict resilience / “real world” adaptive functioning in young adults with 22q11DS.
Limitations
The present study was not without limitations. The smaller number of participants with 22q11DS and control participants who had Time 4 data likely limited our power for detecting smaller effects of childhood EF on young adult outcomes. In spite of our limited sample size, however, several significant effects of EF on developmental outcomes were detected, and the subsample of participants examined in this study was not demographically different from the total sample, suggesting that these results would likely generalize. A second limitation is the number of analyses conducted and the potential for type I error. We chose not to apply a correction for multiple testing because of our small sample size and the potential increase in type II error. A study with low power also has a lower positive predictive value. Thus, these results should be considered preliminary until other groups can independently replicate these findings. A third limitation is that we were unable to examine teacher reports of children’s EF. Thus, we were unable to include information about children’s EF in the school setting, which may differ from their EF observed in other settings. Future research should also consider examining teacher reports to address the limitations of common method variance, as in the present study, parent report is used for both predictor and outcome variables. Having both teacher and parent reports would provide information about convergent validity, which would indicate greater reliability of findings.
Additionally, the present study did not consider the potential impact of treatment. It is possible that participants with 22q11DS received treatment at some point during the 9-year time period in which this study was conducted. Treatment may have impacted young adult outcomes for children with 22q11DS. Likewise, the majority of young adults with 22q11DS (and controls) were living at home in young adulthood yet our analyses did not consider how supportive those home environments may be. Thus, we do not know the extent to which support systems may moderate our findings. Similarly, due to insufficient statistical power, we did not consider that psychiatric symptoms may moderate our results. Thus, we do not know the extent to which our findings may be less driven by 22q11DS and more impacted by psychopathology. Our neuropsychological test battery did not include a performance validity test. Thus, the issue of suboptimal participant effort may have impacted our results. Finally, for the analyses, standardized scores were used for all variables except for the Tower of London. Thus, our Tower of London results may be differentially impacted by the PCA analyses and should be viewed with caution.
CONCLUSIONS
Childhood EF is an important predictor of young adult outcomes among individuals with and without 22q11DS. This has important implications for clinical practice, because it suggests that EF could be a valuable target for treatment in children with 22q11DS. This study provides support for assessing the efficacy of preventive cognitive remediation (CR) interventions for children with 22q11DS, such as the computer-based CR intervention investigated by Mariano and colleagues (Mariano et al., Reference Mariano, Tang, Kurtz and Kates2015, Reference Mariano, Tang, Kurtz and Kates2018). That is, Mariano and colleagues administered a longitudinal, computer-based CR program (Bracy et al., Reference Bracy, Oakes, Cooper, Watkins, Watkins, Brown and Jewell1999) to 21 adolescents with 22q11DS, and observed that participants exhibited significant improvements in working memory, shifting attention, and cognitive flexibility. These improvements were maintained over a six-month period that followed the intervention program.
Findings of the present study suggest improving children’s EF may reduce the likelihood of a variety of negative outcomes in later development. Additionally, parent report of childhood EF was a consistent predictor of young adult outcomes. This finding has important implications for neuropsychologists, because it suggests the information collected through parent-report measures of EF may be more valuable for identifying children at risk for negative developmental outcomes than information collected through behavioral paradigm measures of EF.
ACKNOWLEDGMENTS
The authors have no conflicts of interest to report. This project was funded by the National Institutes of Health, grant number R01MH064824 to Wendy R. Kates, Ph.D. The authors are additionally grateful to the families who participated in the project.
Supplementary materials
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617718000784






