Introduction
Using cryopreserved sperm in in vitro embryo production (IVEP) is useful to increase repeatability during an experiment and sperm from the same ejaculate can be used over a long period of time. However, sperm motility and viability after freezing–thawing vary between straws and individual boars (Holt et al., Reference Holt, Medrano, Thurston and Watson2005; Waterhouse et al., Reference Waterhouse, Hofmo, Tverdal and Miller2006; Yeste, Reference Yeste2017) and are often lower compared with fresh semen. During in vitro fertilization (IVF) the final concentration of all sperm cells per ml during co-incubation with oocytes is usually reported, whereas the percentage total motile or progressively motile sperm cells are often not considered. In vivo, the facilitation of sperm transport through the female reproductive tract is not only due to uterine contractions but, among other things, also due to progressive motility of spermatozoa, which is defined as the straightforward movement in a clear direction. This is supported by the finding that progressive motility had a significant effect on in vivo farrowing rate (Broekhuijse et al., Reference Broekhuijse, Šoštarić, Feitsma and Gadella2012). Moreover, progressive motility has been shown to be of importance during bovine IVF as progressive motility of frozen–thawed sperm showed a good correlation with in vitro pronucleus formation (Tanghe et al., Reference Tanghe, Van Soom, Sterckx, Maes and De Kruif2002) and higher cleavage and blastocyst rates were obtained with high progressively motile sperm compared with low progressively motile sperm (Li et al., Reference Li, Kalo, Zeron and Roth2016).
It is well known that large variations are observed between individual boars when studying IVEP outcomes using both fresh and frozen–thawed sperm (Wang et al., Reference Wang, Niwa and Okuda1991; Xu et al., Reference Xu, Seth, Harbison, Cheung and Foxcroft1996; Almiñana et al., Reference Almiñana, Gil, Cuello, Roca, Vazquez, Rodriguez-Martinez and Martinez2005; Gil et al., Reference Gil, Almiñana, Roca, Vázquez and Martínez2008). It is further shown that the sperm–oocyte ratio affects fertilization and polyspermy rates, and that the optimal ratio even varies between boars (Wang et al., Reference Wang, Niwa and Okuda1991; Xu et al., Reference Xu, Seth, Harbison, Cheung and Foxcroft1996; Gil et al., Reference Gil, Ruiz, Cuello, Vazquez, Roca and Martinez2004, Reference Gil, Almiñana, Cuello, Parrilla, Roca, Vazquez and Martinez2007). In addition to individual boar differences, differences between breeds have been reported for penetration and polyspermy rates (Suzuki et al., Reference Suzuki, Saito, Kagawa and Yang2003). Therefore, it has been suggested that preliminary screening for each individual boar is required to select optimal IVF conditions (Almiñana et al., Reference Almiñana, Gil, Cuello, Roca, Vazquez, Rodriguez-Martinez and Martinez2005; Gil et al., Reference Gil, Almiñana, Roca, Vázquez and Martínez2008). A sperm–oocyte ratio of 1000:1 is often used in porcine IVF (Kidson et al., 2004; Gil et al., Reference Gil, Almiñana, Cuello, Parrilla, Roca, Vazquez and Martinez2007; Martinez et al., 2017). Progressive sperm motility is ∼50% after freezing and thawing in our laboratory and, as this differs between boars and straws, we were interested in performing fertilization with an adjusted sperm–oocyte ratios for all IVF rounds.
The aim of this study was to assess fertilization with a 250:1 and 500:1 ‘progressively motile sperm–oocyte’ ratio using cryopreserved semen from three Duroc and three Landrace boars. It was hypothesized that: (1) a higher 500:1 sperm–oocyte ratio results in higher in vitro fertilization and blastocyst yield but also in an increase in polyspermy compared with a lower 250:1 ratio; and that (2) adjustment to the same number of progressively motile sperm cells per oocyte at fertilization will reduce variation in IVEP results between individual boars.
Materials and methods
Animals and experimental design
Oocytes were collected from random sow ovaries without specified breed in both the luteal and follicular phase of the oestrus cycle. Cryopreserved boar semen was available from six artificial insemination (AI) boars originating from two purebred breeds; three Duroc and three Landrace boars were used. All animals were cared for according to internationally recognized guidelines and regulations for keeping pigs in Norway (The Animal Welfare Act, 10 July 2009 https://www.regjeringen.no/en/dokumenter/animal-welfare-act/id571188/ and Regulations for keeping pigs in Norway, 18 February 2003 https://lovdata.no/dokument/LTI/forskrift/2003-02-18-175). In total, 2456 oocytes were matured in vitro and fertilized with the two sperm–oocyte ratios and semen from the six boars during 14 IVEP rounds. Three to four replicates were carried out per boar and sperm–oocyte ratio. During each IVEP round, a random subset of presumptive zygotes was fixed and stained per boar and sperm–oocyte ratio to assess fertilization (n = 1019 oocytes). The remaining presumptive zygotes (n = 1437) were cultured to assess in vitro embryo development. Data were collected from January to August 2020.
Chemicals and media
All chemicals and reagents were purchased from Sigma-Aldrich (Oslo, Norway) unless stated otherwise. Washing of cumulus–oocyte complexes (COCs) was performed using porcine X medium (PXM), maturation using porcine oocyte medium (POM), fertilization using porcine gamete medium (PGM) and embryo culture using porcine zygote medium-5 (PZM-5) (Yoshioka et al., Reference Yoshioka, Suzuki and Onishi2008). Polyvinyl alcohol in original medium was replaced by 0.4% bovine serum albumin (BSA) in POM and PZM-5 medium and 0.6% BSA in PGM medium. Minor changes were made to the POM medium and the final composition was: 108 mM NaCl, 10 mM KCl, 0.35 mM KH2PO4, 0.4 mM MgSO4.7H2O, 25 mM NaHCO3, 5.0 mM glucose, 0.2 mM Na-pyruvate, 2.0 mM Ca-(lactate). 2.5H2O, 2.0 mM l-glutamine, 5.0 mM hypotaurine, 20 ml/l BME amino acids, 10.0 ml/l MEM non-essential amino acid, 0.6 mM l-cysteine, 0.01 mg/ml gentamicin, 4.0 mg/ml BSA, serum substitute, 10 ng/ml epidermal growth factor, and 50 µM β-mercaptoethanol (Gibco).
Oocyte collection and in vitro maturation (IVM)
Random sow ovaries in the luteal and follicular phase of the oestrus cycle were collected at a commercial slaughterhouse and transported to the laboratory in 0.9% NaCl at 35–38°C. Upon arrival, ovaries were washed with 0.9% NaCl containing 2.5 µg/ml kanamycin and placed in a beaker in a water bath at 30–35°C until follicle aspiration. Follicles with a diameter of 3–8 mm were aspirated 4 h after slaughter using an 18-gauge needle and 10 ml syringe. Oocytes with a compact cumulus and evenly granulated cytoplasm were selected under a Leica MS5 stereomicroscope (Leica Microsystems GmbH, Wetzlar, Germany), washed three times in PXM and once in POM medium, and transferred in groups of 30 oocytes into each well of a Nunc four-well multidish containing 500 µl of pre-equilibrated POM medium. For the first 20 h, COCs were matured in POM supplemented with 0.05 IU/ml porcine follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (Insight Biotechnology Ltd, Wembley, UK), and 0.1 mM dbcAMP. Subsequently, COCs were matured for another 24 h in POM without hormones and dbcAMP. Oocytes were matured at 38.8°C in an humified atmosphere containing 6% CO2 in air.
Sperm preparation and in vitro fertilization (IVF)
Fertilization was performed with cryopreserved sperm from three Duroc and three Landrace boars. Frozen straws from each individual boar originated from the same ejaculate. Each 2.5 ml straw was thawed at 50°C for 50 s (Waterhouse et al., Reference Waterhouse, Hofmo, Tverdal and Miller2006) and diluted in 40 ml Tri-X-cell (IMV technologies, L’Aigle, France) at room temperature (RT). Sperm cells were washed and selected at RT using Percoll® density gradient centrifugation by layering 2 ml of 45% Percoll® on top of 2 ml 90% Percoll®. Finally, 1 ml of semen was carefully placed on top, and the sample was centrifugated at 700 g for 20 min. Supernatant was removed by aspiration, the pellet was resuspended in 4 ml PGM without BSA and centrifuged at 500 g for 5 min. The pellet was then resuspended in 150–200 µl PGM without BSA. Sperm concentration and progressive motility was measured using computer-assisted sperm analysis (CASA) and spermatozoa were diluted to 5 × 105 progressively motile sperm cells/ml in 300 µl PGM with BSA. The COCs were carefully washed once in PGM and groups of 30 oocytes were co-incubated with 15 µl sperm suspension (1 oocyte:250 progressively motile sperm cells, i.e. 1.5 × 104 progressively motile sperm cells/ml) or 30 µl (1 oocyte:500 progressively motile sperm cells, i.e. 3.0 × 104 progressively motile sperm cells/ml) in a final volume of 500 µl PGM per well. After 2 h of co-incubation, oocytes were transferred to a new well with 500 µl PGM medium to remove an excess of sperm cells.
In vitro culture (IVC)
After 4 h co-incubation, presumptive zygotes were denuded of cumulus cells by vortexing for 1 min in 2 ml PXM. The zygotes were washed twice in PXM medium and once in PZM-5 before culture in 500 µl PZM-5 under 400 µl mineral oil (IVF Biosciences, Falmouth, UK) at 38.8°C in an humified atmosphere containing 6% CO2 and 7% O2. At day 4 of culture (fertilization = day 0), PZM-5 medium was refreshed by taking 250 µl out and replacing it with 250 µl new equilibrated PZM medium.
Assessment of sperm motility by CASA
Sperm motility parameters were assessed after Percoll density gradient centrifugation using a Sperm Class Analyzer® version 6.1 (Microptic SL, Barcelona, Spain), equipped with a phase contrast Eclipse Ci-S/Ci-L microscope (Nikon, Japan) and Basler digital camera (Basler Vision Technologies, Ahrensburg, Germany). Per sample, 3 µl was loaded into a pre-warmed Leja-4 chamber slide (Leja Products, Nieuw-Vennep, The Netherlands) and analyzed with a frame rate of 45 frames per second and a minimum of eight microscope fields and 800 cells. Total motility (MOT) was defined as sperm cells with curvilinear velocity (VCL) > 10 µm/s and progressive motility (PROG) with straightness (STR) > 45 %.
Sperm plasma membrane and acrosome integrity by flow cytometry
All analyses by flow cytometry were performed with a Cell Lab Quanta™ SC MPL flow cytometer (Beckman Coulter, Fullerton, USA). Sperm plasma membrane and acrosome integrity, were analyzed after Percoll centrifugation. Sperm samples were diluted to a concentration of 1 × 106 sperm cells/ml and stained with 0.05 μ/ml Lectin peanut agglutinin (PNA) conjugated with Alexa Fluor 488 (PNA-Alexa 488, L21409, Invitrogen) and 0.48 μM propidium iodide (PI) in PBS with 1% guaiacol glycerol ether (GGE) to identify live and dead sperm cells and acrosome-reacted spermatozoa, respectively. Sperm cells were incubated for 10 min at RT before analysis. Acrosome-intact and live spermatozoa (AIL) were recorded and analyzed using Cell Lab Quanta™ SC MPL software (Beckman Coulter, software version 1.0 A).
Sperm DNA fragmentation index (DFI) by flow cytometry
Sperm chromatin integrity was analyzed after Percoll centrifugation using the sperm chromatin structure assay (SCSA) (Evenson and Jost, Reference Evenson and Jost2000; Boe-Hansen et al., Reference Boe-Hansen, Ersbøll, Greve and Christensen2005). Sperm samples were diluted to a concentration of 2 × 106 sperm cells/ml in TNE buffer (10 mM Tris–HCl, 0.1 M NaCl, 1 mM EDTA, pH 7.4) in a final volume of 200 μl. Immediately after dilution, 400 μl acid detergent solution [0.38 M NaCl, 80 mM HCL, 0.1% (w/v) Triton X-100, pH 1.2] was added to denature the samples. After 30 s of incubation at RT, denatured samples were stained with 1.2 ml of 6 μg/ml acridine orange staining solution (A3568, Invitrogen) in a buffer (37 mM citric acid, 0.126 M Na2HPO4, 1.1 µM EDTA and 0.15 M NaCl, pH 6). The samples were run in setup mode for 3 min, after which data acquisition started with 5000 events collected for each sample. The percentage of red (single-stranded DNA) and green (double-stranded DNA) fluorescence was determined using FCS Express 6 flow cytometry software (De Novo Software, USA). The percentage of DFI was calculated based on the fluorescence ratio red/(red + green).
Assessment of fertilization and polyspermy
To analyze fertilization and polyspermy rates, presumptive zygotes were assessed 10–12 h after start of fertilization. During each IVF replicate, one well with zygotes was fixed per sperm–oocyte ratio and boar. The presumptive zygotes were kept overnight in 4% PFA at 4°C, stained the next morning for 5 min in 8 µg/ml Hoechst stain (H-33342, B2261, Sigma) and mounted in 6 µl fluorescence mounting medium (Dako, Glostrup, Denmark) under a coverslip. Pronucleus formation was assessed using a fluorescence microscopy and a Leica SP8 laser scanning confocal microscope. Hoechst staining was evaluated with a 405 nm excitation laser and a 410 to 480 nm emission filter. Oocytes were classified as fertilized when they had one or more swollen sperm heads and pronuclei, and polyspermy was defined as the proportion of zygotes with more than two pronuclei or swollen sperm heads as described in del Olmo et al. (Reference del Olmo, Parrilla, Gil, Maside, Tarantini, Angel, Roca, Martinez and Vazquez2013).
Assessment of embryo development and quality
Cleavage rate at day 2 and blastocyst rates at day 6 and 7 of culture were assessed per well using a Leica DM IL inverted microscope. The rates were defined as the number of cleaved oocytes or blastocysts divided by the total number of oocytes cultured. An embryo with a clear blastocoel was defined as a blastocyst. Day 7 blastocysts were fixed (n = 260) in 4% PFA at RT for 30 min and stained with 8 µg/ml Hoechst stain to assess total blastocyst cell number. Blastocysts that showed apoptotic nuclei with fragments after staining, and in which the total blastocyst cell number was difficult to count, were not included in the analysis.
Statistical analysis
Statistical analysis was performed using SAS v.9.4 (SAS Institute Inc., Cary, NC, USA). Distributions of the means and residuals were assessed to verify normality using Shapiro–Wilk’s test and homogeneity of variance using Levene’s test. Sperm DFI was log transformed to obtain normality before statistical analysis. Differences in fertilization, polyspermy, cleavage rate, blastocyst rate and total blastocyst cell number were studied using a mixed linear model (proc mixed). Sperm–oocyte ratio (250:1 or 500:1) and boar (1:6) were set as fixed effects and IVF week as a random effect as different oocyte materials over different seasons was used. Interactions between sperm–oocyte ratio and boar were not significant, and therefore not included in the models. When analysis of variance (ANOVA) revealed a significant effect, values were compared using the post hoc multiple pairwise-comparison Tukey test. Results are presented as least squares means ± standard error of the mean (SEM) and P ≤ 0.05 was considered statistically significant. Figures were plotted using GraphPad Prism v.9.0 (GraphPad Software, San Diego, USA).
Results
Sperm motility, viability and DNA fragmentation
Sperm parameters after centrifugation are shown in Table 1 for the different boars. A variation in total and progressive motility between boars and straws was observed for the individual sperm samples. However, average total and progressive motility were not significantly different between the boars. Average total motility ranged from 57.1% to 74.5%, of which 38.1–61.0% of the sperm showed progressive motility. Therefore, the number of progressively motile sperm cells per oocyte was adjusted for each sperm sample to obtain either a ratio of 250:1 or 500:1 across all IVF rounds. Furthermore, no significant difference in DFI was observed, while the average percentage acrosome-intact live sperm cells was higher for Duroc boar 2 compared with Landrace boar 2 (P < 0.05).
Table 1. Sperm parameters per boar after Percoll density gradient centrifugation and before adjustment (mean ± standard deviation (SD))
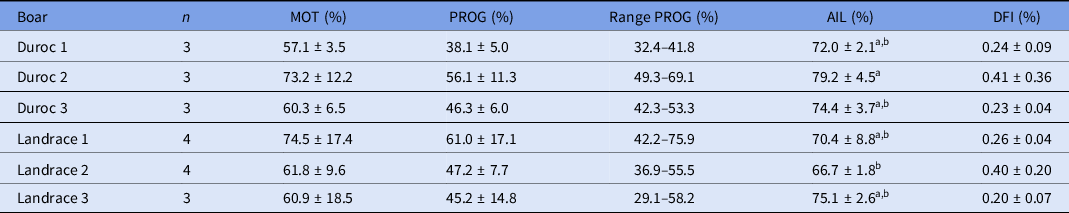
n, number of straws analyzed and used for IVF.
AIL, acrosome intact live; DFI, DNA fragmentation index; MOT, total motility; PROG, progressive motility.
Each sperm sample was evaluated for the percentage of total motile and immotile sperm. Total motility consists of a percentage of progressive motile and non-progressive motile sperm cells.
a,b Values with different superscript letters within a column are significantly different (P < 0.05).
IVEP outcomes
Averages and standard deviations for the IVEP outcomes are presented in Table 2 for each individual boar and sperm–oocyte ratio. Results indicated that Landrace boar 3 had a higher average blastocyst rate for the 250:1 ratio compared with the 500:1 ratio (29.5 % vs 17.1%, respectively), while the other boars had a higher blastocyst rate at the 500:1 ratio. It was observed that blastocysts sometimes started to collapse on day 7, independent from the ratio or boar. Average blastocyst yield on day 7 was 22.4 ± 12.7% and average total blastocyst cell number was 59.8 ± 22.6 cells.
Table 2. Descriptive statistics for fertilization, polyspermy, cleavage and blastocyst formation rates per boar and ‘progressively motile sperm–oocyte’ ratio (mean ± standard deviation (SD))

a Number of presumptive zygotes analyzed for fertilization and polyspermy rates.
b Percentage of fertilized oocytes that were polyspermic.
c Number of zygotes cultured to assess cleavage rate at day 2 and blastocyst formation rate on days 6 and 7.
Cleavage and blastocyst rates were defined as the number of cleaved oocytes or blastocysts divided by the total number of presumptive zygotes cultured.
Sperm–oocyte ratios
A higher fertilization rate and blastocyst formation rate at day 6 (P < 0.05) were observed for the 500:1 ratio compared with the 250:1 ratio, while the polyspermy level was consistent across ratios (Table 3). No significant effect of sperm–oocyte ratio was observed for cleavage rate, blastocyst rate at day 7 or total blastocyst cell number.
Table 3. Effect of ‘progressively motile sperm cell to oocyte’ ratio on fertilization, polyspermy, cleavage and blastocyst formation rates and total blastocyst cell number (least squares (LS) means ± standard error of the mean (SEM))
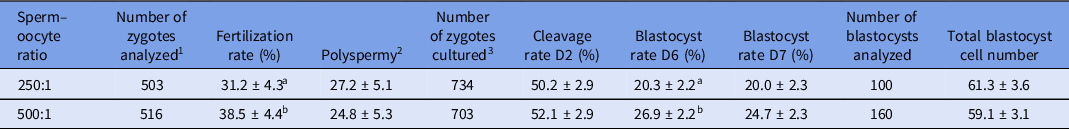
1 Number of presumptive zygotes analyzed for fertilization and polyspermy rates.
2 Percentage of fertilized oocytes that were polyspermic.
3 Number of presumptive zygotes cultured to assess cleavage rate at day 2 and blastocyst formation rate on days 6 and 7.
Cleavage and blastocyst formation rates were defined as the number of cleaved oocytes or blastocyst divided by the total number of presumptive zygotes cultured.
a,b Values with different superscript letters within a column are significantly different (P < 0.05).
Individual boar difference
Differences in IVEP outcomes between boars are shown in Figure 1. A significant effect of boar was observed for fertilization rate (P < 0.05), cleavage rate (P < 0.001) and blastocyst rates (P < 0.001). Fertilization rate (Fig. 1A) and blastocyst rates on day 6 and 7 (Fig. 1D) were significantly lower for Duroc boar 3 compared with Landrace boar 1. Cleavage rate at day 2 was lower for Duroc boar 3 compared with Landrace boars 1 and 3 (Fig. 1C). No significant differences in polyspermy (Fig. 1B) and total blastocyst cell number on day 7 of culture were observed between the boars.

Figure 1. In vitro embryo production outcomes for the different boars for both ratios. (A) Fertilization rate, (B) polyspermy rate, (C) cleavage rate at day 2, and (D) blastocyst formation rate at day 7 per Duroc boar (D1–D3) and Landrace boar (L1–L3). *P < 0.05; **P < 0.01. Results are presented as least squares (LS) means ± standard error of the mean (SEM).
Discussion
The aim of this study was to assess fertilization with a 250:1 and 500:1 ‘progressively motile sperm to oocyte’ ratio using cryopreserved semen from three Duroc and three Landrace boars. To our knowledge this is the first study assessing IVEP outcomes after adjustment for the number of sperm cells based on progressive motility. The 500:1 ratio resulted in a higher fertilization rate and blastocyst percentage on day 6 of culture compared with the 250:1 ratio, but no effect of sperm–oocyte ratio on polyspermy, cleavage rate, blastocyst formation rate on day 7 or total blastocyst cell number was observed. A higher incidence of polyspermy was expected for the highest oocyte-sperm ratio as a decrease in penetration rate and polyspermy is normally observed when the sperm concentration is reduced in IVF as reviewed by Coy and Avilés (Reference Coy and Avilés2010), but this was not the case in our study. One explanation, however, could be that the highest ratio in this study (500:1) was lower compared with other studies on sperm–oocyte ratios, ranging from 2000:1 to 8000:1 and 3000:1 to 50.000:1 (Xu et al., Reference Xu, Seth, Harbison, Cheung and Foxcroft1996; Gil et al., Reference Gil, Ruiz, Cuello, Vazquez, Roca and Martinez2004). On day 7 of culture, no more difference was observed anymore in blastocyst formation between the two ratios. It was noticed that blastocysts sometimes started to collapse from day 6 to day 7 of culture, independent of the boar or ratio, and this could have affected the results. Furthermore, apoptotic nuclei were observed in some of the blastocysts and no hatching was observed. This can indicate suboptimal culture conditions and may even be related to degeneration of polyspermic zygotes. In further studies it will be of interest to use porcine blastocyst medium (PBM) during IVC, as this optimized culture medium is shown to improve embryo quality, development to blastocyst stage and hatching (Mito and Hoshi, Reference Mito, Hoshi and Herrick2019). Additionally, fetal bovine serum has improved hatching (Yoshioka et al., Reference Yoshioka, Suzuki and Rodriguez-Martinez2005). However, a defined or semi-defined medium is preferable as medium supplemented with serum components carry a risk of disease transmission. Embryo transfers with in vitro-produced blastocysts on days 5 and 6 have successfully resulted in liveborn piglets (Mito et al., Reference Mito, Yoshioka, Noguchi, Yamashita, Misumi, Hoshi and Hoshi2015; París-Oller et al., Reference París-Oller, Navarro-Serna, Soriano-Úbeda, Lopes, Matás, Ruiz, Latorre, López-Albors, Romar, Cánovas and Coy2021; Suzuki et al., Reference Suzuki, Misumi, Ozawa, Noguchi, Kaneko, Ohnuma, Fuchimoto, Onishi, Iwamoto, Saito, Nagai and Kikuchi2006). When performing embryo transfers, it is beneficial to get as many blastocysts as possible, but a longer blastocyst culture than needed is unnecessary and might impair embryo quality and increase the risk of embryo hatching. Therefore, it was concluded that the 500:1 sperm–oocyte ratio and culture until day 6 is optimal within our IVF system for both breeds.
Variation in sperm motility between boars and between straws from the same boar was observed. Although adjusted to the same number of progressively motile sperm cells per oocyte for all boars and replicates in this study, differences in IVEP outcomes were still observed between some of the boars. Duroc boar 3 differed most and showed the lowest results, while the best blastocyst results were obtained for Landrace boar 1 with an average blastocyst formation rate of 37.0% on day 6 for the 500:1 ratio. No difference in blastocyst cell count was observed between the boars, but there was variation for all boars. Total blastocyst cell number was relatively high in this study for all boars (on average 59.8 ± 22.6 cells), as 30–45 cells are usually reported for good quality in vitro-produced blastocysts (Gil et al., Reference Gil, Gomis, Angel, Sanchez-Osorio, Maside, Cuello, Parrilla, Roca, Vazquez and Martinez2013; Yoshioka et al., Reference Yoshioka, Uchikura, Suda and Matoba2020; Yuan et al., Reference Yuan, Spate, Redel, Tian, Zhou, Prather and Roberts2017). Average blastocyst yield in our study was lower (22.4 ± 12.7% for all boars) compared with the study that also supplemented IVM medium with serum substitute and reported a blastocyst yield of 40% (Yuan et al., Reference Yuan, Spate, Redel, Tian, Zhou, Prather and Roberts2017). However, findings are similar for the best boar and 500:1 ratio which resulted in a blastocyst yield of 36.2 ± 6.4%. It should be considered that different media have been used that can affect culture success. Interestingly, results indicated that average blastocyst rate for Landrace boar 3 was higher for the 250:1 ratio compared with the 500:1 ratio. This is in line with others who reported differences in optimal sperm–oocyte ratio between boars (Xu et al., Reference Xu, Seth, Harbison, Cheung and Foxcroft1996; Gil et al., Reference Gil, Ruiz, Cuello, Vazquez, Roca and Martinez2004, Reference Gil, Almiñana, Cuello, Parrilla, Roca, Vazquez and Martinez2007) and the fact that preliminary screening for each individual boar is recommended before IVF.
Adjustment for the same number of progressively motile sperm cells present per oocyte did not result in similar and high blastocyst rates among the boars. This suggests that there are factors other than just the number of progressively motile sperm that influence IVEP results. Suzuki and Nagai (Reference Suzuki and Nagai2003) observed that epididymal sperm samples with high total and progressive motility did not always result in high fertilization rates. In the present study, more spermatozoa were added for the samples with a lower progressive motility, which increased the total number of sperm and also the number of immotile or dead sperm cells present during fertilization for the different replicates and boars. Roca et al. (Reference Roca, Martinez-Alborcia, Gil, Parrilla and Martinez2013) indicated that a higher proportion of dead spermatozoa but same number of viable sperm cells in raw semen before freezing negatively affected in vitro fertilization, cleavage and blastocyst formation. The dead sperm cells affect the intracellular reactive oxygen species (ROS) in the viable spermatozoa after freezing and thawing that, at too high levels, led to sperm DNA damage, as shown in different species (Takahashi et al., Reference Takahashi, Keicho, Takahashi, Ogawa, Schultz and Okano2000; Agarwal and Said, Reference Agarwal and Said2003; Simões et al., Reference Simões, Feitosa, Siqueira, Nichi, Paula-Lopes, Marques, Peres, Barnabe, Visintin and Assumpção2013). Interestingly, an in vitro study in bovine showed that DNA damage induced by sperm oxidative stress affected cleavage rate but not blastocyst formation rate or quality (Simões et al., Reference Simões, Feitosa, Siqueira, Nichi, Paula-Lopes, Marques, Peres, Barnabe, Visintin and Assumpção2013). In our study, Duroc boar 3 had significantly lower fertilization, cleavage and blastocyst rates compared with Landrace boar 1, but progressive motility was not lower. In contrast, Duroc boar 1 had generally a lower progressive motility after Percoll density centrifugation and therefore more sperm was added during the IVF rounds compared with the other boars, which have resulted in more immotile or dead sperm cells present during fertilization, but IVEP results were good for this boar. This suggests that a higher proportion of immotile or dead sperm cells did not affect IVEP outcomes in our study. In cattle it has been observed that after IVF, only the percentage of live spermatozoa was associated with fertilization outcomes (Tanghe et al., Reference Tanghe, Van Soom, Sterckx, Maes and De Kruif2002). Furthermore, several studies have observed positive correlations between progressive motility and penetration rate (Xu et al., Reference Xu, Seth, Harbison, Cheung and Foxcroft1996; Gadea and Matás, Reference Gadea and Matás2000) while others did not (Suzuki et al., Reference Suzuki, Mori and Shimizu1996; Popwell and Flowers, Reference Popwell and Flowers2004). The sperm parameters in the present study were assessed as means per sperm sample but, lately, evaluation has been based on individual sperm kinetics and subpopulations. In bovine it was shown that the population characterized with rapid and progressive motility had the greatest effect on IVEP outcomes (Peres Campanholi et al., Reference Peres Campanholi, Garcia Neto, Basso, de Agostini Losano, Perez Siqueira, Nichi, Ortiz D’Avila Assumpção, Afonso de Freitas, Paro de Paz, Ferraudo, Morato Monteiro and Unno Gimenes2021) and was more resistant to cryopreservation (Muiño et al., Reference Muiño, Tamargo, Hidalgo and Peña2008). Therefore, it could be of interest to evaluate sperm subpopulations with specific movement patterns by a cluster analysis for individual boars and breeds in relation to IVEP outcomes.
In conclusion, fertilization with the 500:1 ratio resulted in a higher fertilization rate and blastocyst yield on day 6, while polyspermy did not increase with the higher sperm–oocyte ratio. Differences in IVEP outcomes were still observed between the individual boars although adjusted for progressive motility. Promising blastocyst yields and high blastocyst cell numbers were obtained with cryopreserved sperm from both Duroc and Landrace boars.
Financial support
This work was partly supported by The Research Council of Norway (grant no. 283804).
Conflict of interest
The authors declare no conflict of interest.
Ethical standards
Oocytes were collected from routinely slaughtered animals, a procedure that did not require ethical approval.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.






