Maternal anaemia continues to be a major barrier to women's health, social development and economic growth, especially in the developing world. The WHO estimates that, globally, anaemia affects 42 % (56·4 million) of pregnant women and 30 % (468·4 million) of non-pregnant women (including adolescent girls). In Africa, 57 % (17·2 million) of pregnant women and 48 % (70 million) of non-pregnant women (including adolescent girls) are anaemic(1). In Sub-Saharan Africa, and more particularly in the West and Central Africa Region (WCAR),Footnote † high rates of anaemia continue unabated in women throughout their reproductive years. In WCAR, according to WHO classification, the prevalence of anaemia among women of reproductive age is of severe public health significance; i.e. at any point in time at least 40 % of women (15–49 years old) are anaemic(1).
These high rates represent significant constraints for achieving some of the Millennium Development Goals such as eradicating hunger and poverty (Goal 1), reducing child mortality (Goal 4) and improving maternal health (Goal 5). The adverse consequences of maternal anaemia include fatigue, decreased work capacity and poor pregnancy outcomes such as preterm birth, low birth weight, and increased risk of maternal death both during delivery and the postpartum period(Reference Rasmussen2, Reference Cogswell, Parvanta and Ickes3). Even though iron/folic acid (IFA) supplementation for pregnant women is policy in all countries of WCAR, programme implementation has been very irregular; and therefore the prevalence of anaemia among women in this region remains unacceptably high.
In recognition of the failure of such programmes globally, with a few exceptions, the international community recently endorsed a set of guidelines for the integrated control of anaemia(4). The effective implementation of these guidelines is essential, especially in areas where the prevalence of anaemia is known to be a public health problem and the causes of anaemia are multiple and coexist. However, this integrated strategy is seldom considered in the training curricula of health professionals or implemented by the health systems in most countries.
The objective of the present paper is to review the extent, severity and determinants of anaemia among women (pregnant, lactating and non-pregnant) in the countries of WCAR, and hence to inform policy makers and programme planners in the region about the consequences of anaemia on child and maternal survival and development, and the feasibility of sound policy and programme actions for the control of anaemia among women of reproductive age.
Materials and methods
Data presented in the current paper were obtained from the public domain of the ORC Macro MEASURE Demographic and Health Surveys (DHS) website using its STATcompiler program, national nutrition surveys, oral and technical communications at regional meetings held in West and Central Africa, a PubMed search for studies published in scientific journals, WHO and UNICEF databases, and through collaborators.
More specifically, all countries with a national anaemia survey were identified. The final report of the most recent DHS was scrutinized for information on: the prevalence of anaemia (measured by HemoCue®) in pregnant (Hb < 11 g/dl), lactating, non-pregnant/non-lactating women (Hb < 12 g/dl), women's nutritional status and intake of micronutrient supplements, and women's use of prenatal care services and commodities for malaria control. In addition, the proceedings of regional meetings organized by the WHO on anaemia control were used, as appropriate, and unpublished reports were obtained from collaborators. Finally, a thorough search was conducted on UNICEF and WHO databases and on PubMed using combinations of the following keywords: ‘anaemia’, ‘iron deficiency’, ‘pregnancy’, ‘hookworm’, ‘Schistosoma haematobium’, ‘malaria’, ‘Plasmodium falciparum’ and country names. This search was limited to 15 years, i.e. between 1993 and 2008. The data obtained were tabulated or presented in charts to show the results. We provide a personal analysis of the data reported and discuss their policy and programmatic implications for the region.
Results
Prevalence of maternal anaemia in West and Central Africa Region
Fifteen of the twenty-four countries in WCAR have conducted a national survey or a demographic and health survey (DHS) that includes the assessment of Hb concentration in women. These are: Benin, Burkina Faso, Cameroon, Central African Republic, Congo, Democratic Republic of the Congo, Gambia, Ghana, Guinea, Liberia, Mali, Niger, Nigeria, Senegal and Sierra Leone. Table 1 summarizes the data available on anaemia in women in those countries. For other countries of the region without a national survey or a DHS, but with regionally representative data, the most recent WHO estimates(1) are shown in Table 2.
Table 1 Prevalence (%) of maternal anaemia (age group: 15–49 years) in West and Central Africa countries with national or Demographic and Health Surveys
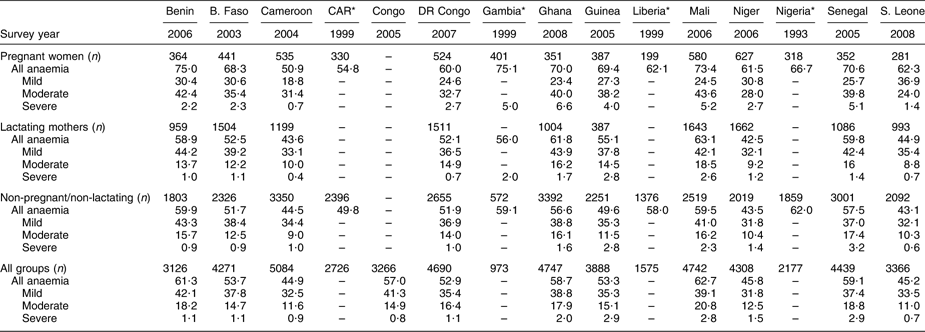
B. Faso, Burkina Faso; CAR, Central African Republic; DR Congo, Democratic Republic of the Congo; S. Leone, Sierra Leone.
All anaemia (Hb < 11·0 g/dl for pregnant women and <12·0 g/dl for non-pregnant women); mild anaemia (Hb = 10·0–10·9 g/dl for pregnant women and 10·0–11·9 g/dl for non-pregnant women); moderate anaemia (Hb = 7·0–9·9 g/dl); severe anaemia (Hb < 7·0 g/dl).
Source: Demographic and Health Surveys and *WHO estimates (WHO, 2008).
Table 2 Prevalence of maternal anaemia (age group: 15–49 years) in West and Central Africa countries without Demographic and Health Surveys but regionally representative data
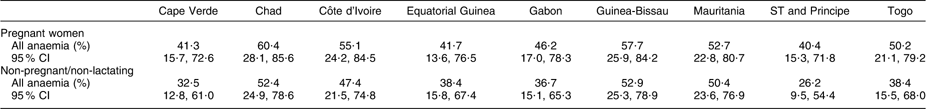
ST and Principe, Sao Tome and Principe.
All anaemia (Hb <11·0 g/dl for pregnant women and <12·0 g/dl for non-pregnant women).
Source: WHO estimates (WHO, 2008).
The prevalence of anaemia among pregnant women (Hb < 11 g/dl) was greater than 50 % in all countries, with the highest rates observed in Benin, Gambia, Mali and Senegal (>70 %) and the lowest in Cameroon (51 %). Gambia, Mali and Senegal had the highest prevalence of severe anaemia (Hb < 7 g/dl) among pregnant women (∼5 %).
The prevalence of anaemia in non-pregnant women (lactating and non-pregnant/non-lactating) was also high in all WCA countries (>40 %) but remained consistently lower than the prevalence among pregnant women.
Within countries, the prevalence of anaemia among women varied by living setting (rural v. urban), women's age, education and parity. These variation correlates differed between countries. For example, women living in rural areas were often more affected, except in Benin. Anaemia prevalence was higher among non-educated women in Burkina Faso and Mali but not in Benin, Ghana and Cameroon. With respect to age, anaemia was more frequent among 15–19-year-old women in Ghana, 20–39-year-old women in Benin, 25–29-year-old women in Cameroon and 35–39-year-old women in Burkina Faso and Mali. In addition, anaemia prevalence was higher among women with one child in Cameroon and Mali whereas this was observed among women with two or three children in Burkina Faso and six or more children in Ghana and Benin.
Determinants of maternal anaemia in West and Central Africa Region
The high prevalence of anaemia described above is reflective of the large extent of nutritional deficiencies (micronutrient and macronutrient), malaria and helminth infections, women's reproductive life (high parity and short birth intervals) and limited access, poor quality and utilization of prenatal health services.
Fe deficiency is the most common cause of anaemia worldwide, and very likely a primary cause of maternal anaemia in WCAR. However, in this region, the extent of Fe deficiency and its contribution to anaemia in women is not well documented. To our knowledge, there are no nationally representative data on the prevalence of Fe deficiency among women of childbearing age in WCAR, except in the Gambia where low ferritin values, i.e. <10 μg/l, were observed in 26 % of pregnant women(5). Most often the prevalence of anaemia (Hb < 11 g/dl) is used as a proxy for Fe deficiency among pregnant women.
In the absence of national data, we report the results of localized research studies published in the scientific literature that describe approximately the extent of Fe deficiency in some countries and portray the big picture of the problem in the region. Table 3 summarizes the major findings of those studies(Reference Prual, Galan and De Bernis6–Reference Isah, Fleming and Ujah10) (also J Kate, unpublished results). It shows that Fe deficiency is prevalent as expected. It also shows that the deficiency is not limited to pregnant women.
Table 3 Prevalence of iron deficiency (serum ferritin or serum iron <12 μg/l) in women in selected countries of West and Central Africa
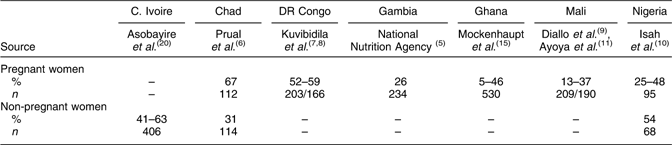
C. Ivoire, Côte d'Ivoire; DR Congo, Democratic Republic of the Congo.
Multiple micronutrient deficiencies also are likely common in the selected countries. This is evidenced by the high prevalence of anaemia (a proxy for Fe deficiency) and the frequent reporting of night blindness (a proxy for vitamin A deficiency) among women in these countries. The reported prevalence of night blindness varies from 6·0 % in Cameroon to 17·8 % in Guinea (Table 4). The highest prevalences are observed in rural areas where the majority of women live. In addition, widespread self- and culturally imposed food restrictions and economic constraints limit dietary diversification in this region. In Mali, for example, 45 % of pregnant women are subject to food restrictions(Reference Ayoya, Spiekermann-Brouwer and Traoré11).
Table 4 Prevalence of night blindness during the last pregnancy among women in selected countries of West and Central Africa
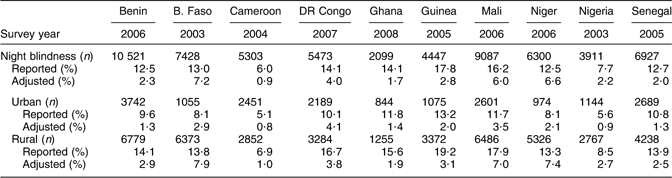
B. Faso, Burkina Faso; DR Congo, Democratic Republic of the Congo.
Source: Demographic and Health Surveys.
The prevalence of chronic energy deficiency as defined by low BMI (BMI < 18·5 kg/m2) among women in the selected countries of WCAR varied from 7 % in Cameroon to 21 % in Burkina Faso. Burkina Faso and Mali (13 %) had the highest rates among the countries presented. In all of these countries, the prevalence of low BMI is higher among younger women (15–19 years old), those without any education and in rural areas.
The effect of all of these nutritional factors on anaemia in women is exacerbated by poor reproductive health and socio-cultural conditions and by the frequency and endemicity of chronic infections and inflammations caused by infectious diseases and worms.
As in other African regions, malaria contributes significantly to the high prevalence of anaemia observed in WCAR, especially among pregnant women. For example, in Mali, during the dry season, a period of low transmission, malaria was found in 11 % of pregnant women cared for in a community health centre(Reference Ayoya, Spiekermann-Brouwer and Traoré11). The disease was associated with anaemia in pregnant women(Reference Bouvier, Doumbo and Breslow12) and contributed 32 % of anaemia cases in this population(Reference Ayoya, Spiekermann-Brouwer and Traoré11). In Gabon, the presence of P. falciparum in peripheral blood was highly associated with anaemia (OR = 2·38, 95 % CI 1·4, 4·05, P < 0·001)(Reference Bouyou-Akotet, Ionete-Collard and Mabika-Manfoumbi13). In Nigeria, P. falciparum was observed in 27 % of pregnant women of whom 60 % were anaemic(Reference Fleming, Ghatoura and Harrison14). In Ghana, P. falciparum was found in 63 % of pregnant women tested and malaria was a major determinant of pregnancy anaemia(Reference Mockenhaupt, Rong and Günther15). In the Democratic Republic of the Congo, malaria was associated with pregnancy anaemia in 40 % of cases(Reference Kalenga, Nyembo and Nshimba16). In Cameroon, 37·0 % of the anaemia cases in pregnant women were associated with maternal malaria parasitaemia while 37·3 % were associated with placental malaria parasitaemia(Reference Achidi, Kuoh and Minang17). In Senegal, the incidence of malaria attacks was, on average, 4·2 times higher during pregnancy than before(Reference Diagne, Rogier and Cisse18). In Burkina Faso, malaria infection was diagnosed in 29 % of pregnant women attending antenatal care clinics(Reference Singer, Newman and Diarra19). In four different regions of Côte d'Ivoire where malaria is endemic, a study showed a prevalence of Fe deficiency and Fe deficiency anaemia in women of 41–63 % and 20–39 %, respectively(Reference Asobayire, Adou and Davidsson20).
The risk of anaemia associated with malaria appears to vary with season. For example in Mali, the risk of anaemia among infected pregnant women is higher during the dry season than during the rainy season (OR = 3·43, 95 % CI 1·09, 10·07). This is likely because pregnant women infected at the end of the rainy season carried their latent infection during the full course of the dry season. This risk also varies with the stage of pregnancy and age, the primigravidae and the youngest women being the groups most at risk of malaria and anaemia(Reference Achidi, Kuoh and Minang17, Reference Dicko, Mantel and Thera21).
In WCAR, helminth infections are widespread. Among the worm infections, hookworm and schistosomiasis are those most known to cause anaemia in man. However, in WCAR, data on their contribution to anaemia in women of childbearing age are lacking or limited. Studies in Mali showed that 8 % and 23 % of urban pregnant women had hookworm and S. haematobium, respectively. In this setting, hookworm and S. haematobium infections contributed 10 % and 13 %, respectively, of the anaemia in women attending a community health centre in Bamako(Reference Ayoya, Spiekermann-Brouwer and Traoré11). In the Democratic Republic of the Congo, anaemia associated with intestinal parasitism (hookworm and Ascaris spp.) was found in 9 % of pregnant women(Reference Kalenga, Nyembo and Nshimba16). In Gambia, hookworm was the cause of anaemia in the majority of cases in non-pregnant adult women(Reference Knight22). In Nigeria, hospital-based studies documented hookworm in 14 % of pregnant women(Reference Egwunyenga, Ajayi and Nmorsi23). In Ghana, a study found that 7 % of women were infected with S. haematobium (Reference Richter, Wagatsuma and Aryeetey24).
Programmes for the control of maternal anaemia in West and Central Africa Region
Supplementation with IFA is a national policy widely adopted among WCAR countries to address anaemia during pregnancy (prevention and treatment). Most often IFA tablets (60 mg of Fe and 400 μg of folate) are prescribed for pregnant women within health facilities from first contact to delivery or 2 months thereafter. These tablets should be taken daily. In spite of this, a large proportion of women (more than 50 % in some countries) do not take Fe supplements during pregnancy (Table 5). The percentage of women not receiving any Fe tablet during the pregnancy period is often almost two or three times higher in rural than urban areas and is higher among women without any education. The reasons for not taking Fe supplements are numerous. However, the most important one is the shortage of Fe tablets(Reference Miaffo, Some and Kouyate25–Reference Vangeenderbuysen and Souley29), a recurrent problem in almost all countries of WCAR because of logistic problems (storage capacity and timely supplies). The shortage may result in limited availability of and accessibility to low-cost Fe tablets for women. For example, studies in Mali reported that Fe tablets are often not available within health services(Reference Ayoya, Spiekermann-Brouwer and Traoré11) (VM Aguayo, unpublished results). There are also other factors contributing to poor compliance such as inadequate or lack of counselling about potential side effects and how to manage them (VM Aguayo, unpublished results).
Table 5 Percentage of women who received vitamin A capsules in the postpartum period and iron + folic acid supplement intakes among mothers in some countries of West and Central Africa
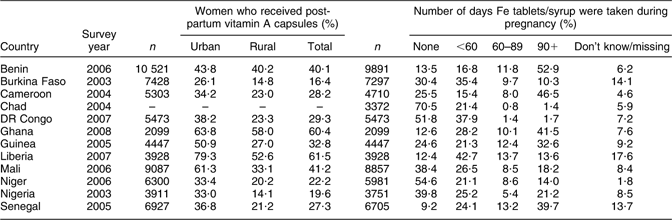
DR Congo, Democratic Republic of the Congo.
Source: ORC Macro, MEASURE DHS STATcompiler, http://www.measuredhs.com,25April2009.
Additional approaches for addressing micronutrient deficiencies – and anaemia indirectly – among women are implemented in the region. Vitamin A (60 000 μg; 200 000 IU), an important micronutrient known to play a key role in erythropoiesis, is recommended in the early postpartum period along with the promotion of vitamin A-rich foods throughout the life cycle. However, even for women delivering in health clinics, the majority do not get vitamin A capsules let alone those delivering at home (Table 5). This is more pronounced in rural areas and among women with no schooling. Similarly, the consumption of vitamin A-rich foods is often greater in urban than rural areas and lower among women with no education. Large-scale food fortification programmes have advanced greatly in the last 5 years in the region. For example, vitamin A fortification of cooking oil (in at least eight countries) and flour fortification with IFA (in one country) are currently being implemented to combat micronutrient deficiencies and anaemia.
Malaria control is being promoted as a complementary approach to IFA supplementation for controlling pregnancy anaemia, among other adverse outcomes, in malaria-endemic areas, hence in WCAR. Until recently, weekly administered chloroquine was the first-line drug for malaria prevention during pregnancy in this region. This policy changed swiftly in several countries (Benin, Burkina Faso, Cameroon, Côte d'Ivoire, Democratic Republic of the Congo, Gabon, Ghana, Guinea, Mali, Niger, Nigeria, Sierra Leone, Senegal and Togo) in favour of the intermittent preventive treatment using sulfadoxine–pyrimethamine (IPT/SP) because resistance to chloroquine became rampant. Nevertheless, the use of IPT/SP was extremely low at the time the surveys were conducted (Table 6). In general, preventive malaria chemotherapy was sought more by women in urban areas and those with the highest education level.
Table 6 Use of antimalarial drugs by pregnant women in selected counties of West and Central Africa
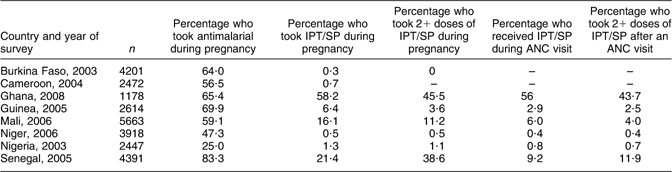
IPT/SP, sulfadoxine–pyrimethamine; ANC, antenatal care.
Source: ORC Macro, MEASURE DHS STATcompiler, http://www.measuredhs.com, 7 July 2009.
Long-lasting insecticide-treated nets, now considered to be the most powerful malaria control tool developed since the advent of indoor residual spraying and chloroquine, were also an important component of national malaria control strategies in WCAR.
The control of helminths is another complementary and cheap strategy to IFA supplementation for controlling anaemia. The landscape of control of helminths has changed quite considerably in several countries in the region (including in Burkina Faso, Mali, Niger and Sierra Leone) with the advent and rapid extension of integrated control programmes for Neglected Tropical Diseases. These programmes potentially have a considerable impact on anaemia. However, nationally representative data on the control of helminths in WCAR women are lacking to our knowledge.
Discussion
The rates of anaemia among women of reproductive age, and especially among pregnant women, in countries of WCAR as reported in a DHS or a national survey are unacceptably high. This is supported by findings from small-scale studies showing that maternal anaemia is a problem that is not limited only to the region's countries with nationally representative data.
Challenges have been reported in ensuring sufficient reliability and accuracy when the HemoCue technique is used to assess Hb levels. The major factors behind those challenges have been shown to be related to sample collection and analysis techniques. There is global evidence showing that the HemoCue method gives highly accurate results(Reference Lardi, Hirst and Mortimer30) and provides an adequate estimate of population anaemia prevalence(Reference Neufeld, Garcia-Guerra and Sanchez-Francia31). DHS follow comprehensive procedures for ensuring rigorous sample collection and analysis developed for use of the HemoCue(Reference Burger and Pierre-Louis32), use various quality control measures and have strong training and supervision components. It is therefore our opinion that the data quality in these surveys is satisfactory and allows a good comparability over time and among countries in WCAR.
Interestingly, WCAR is a region where socio-economic and climatic differences have no apparent association with the prevalence of anaemia during pregnancy across countries. Anaemia proportions are quite similar between the Sahel countries (especially the landlocked ones) supposedly more at risk and the non-Sahel countries with better access to foods and better availability of prenatal health services. This strongly suggests an urgent need for better understanding the local causes of anaemia in these countries, and also for sound public health programmatic and budgetary strategies with appropriate targeting, implementation, monitoring and evaluation in this region.
In terms of targeting, nutrition-related programmes need to focus on and reach the priority groups to achieve the desired results (children under 2 years of age, adolescent girls, and pregnant and lactating women). Adolescent girls represent a particularly important group because nutritional deficiencies at this age have far-reaching implications. Anaemic adolescent girls are more likely to be future anaemic adult mothers. They often have lower pre-pregnancy weight, lower pregnancy weight gain, are at a higher risk of death from haemorrhage at delivery and are more likely to deliver low-birth-weight newborns, thereby perpetuating the vicious cycle of poor nutrition, growth and development. Unfortunately, adolescent girls have been neglected so far by policy makers and programme planners in WCAR.
As reported in the previous section, the prevalence of anaemia among women in WCAR is greater than the threshold of 40 % suggesting that Fe deficiency is likely a severe public health problem at the population level. This is because of chronically inadequate dietary Fe intake in this region caused by poor consumption of animal-source foods. Physiological demands for this essential element imposed by fetal needs and maternal blood volume expansion during pregnancy heighten these inadequate Fe intakes. Furthermore, the absorption of dietary Fe and the utilization of endogenous and exogenous Fe are also influenced adversely by common states of chronic infection and inflammation caused by parasites, which are prevalent in WCAR.
The contribution of vitamin A deficiency to anaemia has been documented. In WCAR, many pregnant women, especially those living in rural areas, suffer from night blindness, a clinical sign of vitamin A deficiency. Indeed, the adjusted prevalence of night blindness among pregnant women in some countries of WCAR is greater than the cut-off point of 5 % indicating that vitamin A deficiency is a public health problem in a population. In a study in rural Gambia, Bates et al.(Reference Bates, Villard and Prentice33) showed that women's plasma retinol levels are significantly lower than those observed in a group of pregnant and lactating women living in the UK. In addition, plasma retinol is significantly higher among pregnant and lactating Gambian women supplemented with 650 μg vitamin A daily(Reference Villard and Bates34).
Parasitic infections (malaria, hookworm and schistosomiasis) clearly play an important role in the occurrence and severity of anaemia among women in WCAR. This assertion is based on the existing evidence from small-scale studies in the region and on the abundant and compelling literature showing that regular parasite control reduces anaemia in women of reproductive age(4). Therefore, the findings reported previously and those of the global research agenda on the benefits of malaria control and deworming for women strongly suggest that appropriate measures should be taken urgently by policy decision makers and programme implementers in collaboration with researchers and civil societies to address parasites in all countries of the region. Malaria control strategies during pregnancy are well defined and commonly implemented. Deworming is possible and feasible because anthelmintic therapy is efficacious, inexpensive, and safe to administer to pregnant women(Reference Allen, Crompton and de Silva35–Reference Olds37). This could be done through systematic treatment of pregnant women for intestinal and urinary parasites during prenatal care visits in their second or third trimester, and through mass campaign distributions of anthelmintics to women of childbearing age following WHO recommended dosages. Such a programmatic strategy, in addition to its enormous impact on the health and survival of both pregnant women and their babies, will position the region to meet the Millennium Development Goals in several ways(4).
The effectiveness of ongoing control programmes for anaemia in the region is seriously hindered by inadequate supply and distribution systems; absence or scarcity of scientific and programmatic information; poor compliance with supplements (when available); inadequate or lack of counselling on the supplementation; low or non-existence of Fe-fortified foods; absence of systematic diagnosis and treatment procedures for helminth infections during antenatal care services; lack of appropriate knowledge by health providers about anaemia; and lack of or insufficient political commitment. The lack of political commitment explains the absence of harmonized reproductive health and nutrition procedures and guidelines for anaemia control in countries. It also is characterized by weak health systems that are not capable of implementing policies and guidelines when they exist (no laboratories, not enough qualified and motivated staff, and no supplies).
Because Fe tablets are distributed to women during their visits to prenatal care clinics, this inherently predisposes those not attending the health centres to be left out. This distribution system limits proper supplementation because women must make multiple visits to the centres to get the recommended number of tablets, which is unlikely in most situations. The health centres also are not always available particularly in rural areas, thus systematically excluding many women from receiving the tablets. Even when health centres exist, women's financial constraints and/or dependency on their husbands can be other barriers to attendance and access to Fe supplements.
Information on the effectiveness of anaemia programmes is scarce or difficult to find because of the lack of well-established monitoring and evaluation systems. In addition, programme implementers report many constraints and few successes of large-scale experiences have been reported. Furthermore, operations research, which is necessary to understand how to overcome practical barriers for successful implementation of programmes, is often neglected.
Good access to and utilization of family planning services to reduce parity and increase birth intervals is crucial for women to maintain health and replenish stores of Fe and other minerals and vitamins between pregnancies. Such utilization and access are, unfortunately, not the norm in the countries included in the present paper. This clearly suggests a strong collaboration need between nutrition and women's reproductive health services. The available data show that women in urban areas and those with formal education seek more family planning services. This is probably because they are more exposed to messages and marketing spots emphasizing the benefits of these services; they understand those benefits; they are more empowered to control their own bodies; and they face less stigma and cultural pressure. Furthermore, their socio-economic status may allow them an easier access to various contraceptive methods. This highlights the need for adjusting and/or strengthening current strategies (information, education, communication, distribution, access) to reach the maximum of women, and more particularly the underprivileged.
The above underscores that the problem of anaemia among women in WCAR is complex and addressing it effectively undoubtedly requires multifactorial and multisectoral approaches. This integrated approach should appropriately use the window of opportunity that prenatal care visits offer. Failing to do so will undermine the development of the region and exacerbate anaemia consequences on maternal and child health, survival and well-being.
The costs of anaemia on maternal health are indeed enormous. Anaemia during pregnancy (and childbearing age) places affected women at greater risk of pre- and postpartum ill health. It lowers their resistance to infections(Reference Stoltzfus38), increases their morbidity and mortality(Reference Brabin, Hakimi and Pelletier39–Reference Geelhoed, Agadzi and Visser41) and reduces their work performance and productivity(Reference Haas and Brownlie42). The mortality impact of maternal anaemia is important and increases with lower Hb levels. For example, the risk of death for women with Hb at 3–4 g/dl ranges from 10 to 30 %; and there is a continuous relationship between maternal mortality and Hb concentration in the range of 5–12 g/dl(Reference Stoltzfus, Mullany and Black43). This risk applies to most pregnant women because the great majority of them have Hb levels in this range. From policy and programmatic points of view, this is of public health significance because it shows that mild and moderate anaemia, not just severe anaemia, are important to women's health.
Anaemia also has been associated with greater risk of preterm delivery and low birth weight(Reference Lone, Qureshi and Emanuel44), thus likely explaining in part the magnitude of low birth weight in the region (Fig. 1). Figure 1(45) shows that the prevalence of low birth weight is high and confirms that many of the region's countries exceed the internationally recommended cut-off levels of low birth weight (incidence rate >15 %) which should trigger public health actions. Population-wide interventions aimed at preventing low birth weight at term (including anaemia control) are therefore urgently required because this condition also contributes substantially to child morbidity and mortality, poor growth and cognitive development, and chronic diseases later in life. For example, recent evidence from Niger shows that prenatal multiple micronutrient supplements have a significantly higher impact on birth weight than IFA alone(Reference Zagré, Desplats and Adou46).

Fig. 1 (colour online) Estimates of the incidence of low birth weight in West and Central Africa (WCA; DR Congo, Democratic Republic of the Congo; CAR, Central African Republic); there are no estimates for missing countries of the region. Source: UNICEF & WHO(45)
Anaemia alters mother–child interactions and is strongly associated with mothers’ depression, fatigue, stress and cognitive functioning during the postpartum period. Anaemic mothers are usually more ‘negative’ towards their babies, engage less in goal setting and are less ‘responsive’ to their infants(Reference Perez, Hendricks and Beard47). They also appear to be more anxious, stressed, tired and depressed after delivery(Reference Beard, Hendricks and Perez48).
The public health consequences of maternal anaemia on child outcomes are also very high. Evidence is available that anaemic mothers are more likely to give birth to anaemic infants. Infants born to anaemic mothers experience more growth faltering and are developmentally delayed in hand–eye movement and overall cognitive quotient(Reference Perez, Hendricks and Beard47). They also are at higher risk for intra-uterine fetal death(Reference Lone, Qureshi and Emanuel44), long-lasting adverse effects on auditory and visual system functioning(Reference Algarin, Peirano and Garrido49) and poor behavioural outcomes, which are only partially irreversible unless corrected early(Reference Lozoff, Jimenez and Wolf50, Reference Allen51).
The persistently high rates of anaemia among women in WCAR and the consequences of anaemia described above could be partly prevented if the quality of prenatal care services is improved. Unfortunately, the region is characterized by a limited availability of health centres, especially in rural areas, a lack of well-qualified and sufficient health workers, and a scarcity of good diagnosis capabilities. This is worsened by the majority of women's financial constraints to seek antenatal care even where it can be provided. Therefore, it is not surprising that the DHS document striking rates of low and late attendance, poor accessibility and utilization of health clinics by women in several countries (Fig. 2) that unquestionably result in low coverage and poor quality of antenatal care.
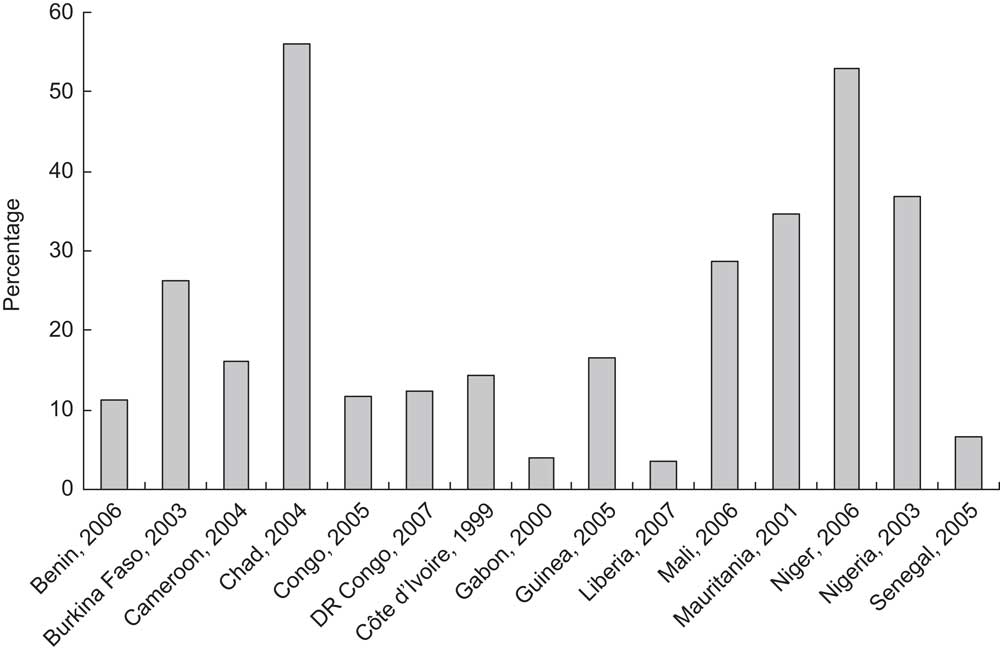
Fig. 2 Percentage of women with no antenatal care during pregnancy in selected countries of West and Central Africa (DR Congo, Democratic Republic of the Congo). Source: ORC Macro, MEASURE DHS STATcompiler, http://www.measuredhs.com,16July2009
Furthermore, there is a lack of adequate and effective strategies to reach women not in contact with health services during pregnancy, hence a need to expand coverage through outreach antenatal care while ensuring quality of the essential package provided. Expanding coverage requires the training of a critical mass of community health workers or volunteers and an increased supply and availability of Fe supplements at each level of the health system. It also requires a community-based distribution system of the supplements by health workers or volunteers and private sector sales by drug vendors and small shops. The existing family planning extension channels can be used to this end. This activity should be done together with the promotion of other services addressing anaemia, such as large-scale food fortification programmes, modern family planning methods to delay and space births, control of malaria and other parasites, and improvement of dietary intake.
A promising approach in the fight against anaemia during pregnancy is the focused antenatal care, which offers an opportunity for strong interpersonal communication component including nutrition counselling. Some of the countries in WCAR are piloting this approach and are actively promoting additional ways of reducing blood loss at delivery among women, which include early initiation of breast-feeding, exclusive breast-feeding and active management of the third stage of delivery.
Another promising approach is the use of multiple micronutrients during pregnancy. For example, in Mali, women had better adherence to the multiple micronutrient supplements and a more positive perception about the benefits to their health and that of their newborns than they did for Fe(Reference Zagré, Desplats and Adou46, Reference Aguayo, Koné and Bamba52). In Niger, multiple micronutrients and Fe were both effective in reducing low birth weight but the multiple micronutrients had slightly more positive effects on this outcome(Reference Zagré, Desplats and Adou46). In both countries, data show that compliance (irrespective of the supplement) was high even with minimum but adequate and easily understandable information and counselling during prenatal care services(Reference Zagré, Desplats and Adou46, Reference Aguayo, Koné and Bamba52).
There are currently unprecedented windows of opportunity and a great potential to improve these prenatal care services. For example, the essential nutrition actions are well defined and commonly known in the region. This represents an excellent opportunity for governments and their development partners to support health services to strengthen the nutrition activities already offered in their service packages, and include those missing. Among the latter is the addition to the current preventive package (Fe + antimalarial) of anthelmintics (albendazole and/or praziquantel). The available WHO recommendations and guidelines on the use of anthelmintic chemotherapy for women of reproductive age (including pregnant women) represent powerful tools in the hands of health professionals and programme managers that can be used and adapted to the prevailing regional, national and local contexts and the resources available, to build the anthelmintic component of an integrated strategy to prevent or address anaemia among women.
The West African Health Organization lately engaged in major activities aimed at reducing anaemia (and Fe deficiency) by 20 % by 2010 in the countries of the Economic Community of West African States through an integrated approach. This offers an important institutional support and an additional opportunity for leveraging resources and implementing the strategy in the region.
Conclusion
The prevalence of moderate to severe maternal anaemia in WCAR is extremely high and varies little among countries. The causes of anaemia among women of childbearing age in this region are multiple and likely interlinked; therefore the consequences of anaemia on maternal and child health, survival and development are probably enormous. Programmes for controlling maternal anaemia in the region have shown significant limitations and need substantial improvements. We believe that the control of anaemia among adolescent girls should be given special attention, and an integrated multisectoral intervention approach recognizing and addressing together the multiple causes of maternal anaemia should be implemented urgently. It is possible and feasible to make progress; however, this calls for unprecedented, historical and stronger political will and commitment that put maternal health at the centre of the development agenda.
Acknowledgements
Source of funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Disclaimer: The named authors alone are responsible for the opinions and views expressed in this publication, which do not necessarily reflect the official position of UNICEF. Conflict of interest declaration: The authors declare no conflict of interest. Author contributions: M.A.A. designed the study and wrote the first draft of the manuscript. M.A.B., N.M.Z. and F.T. reviewed the first draft and all authors approved the final version. Acknowledgements: The authors would like to thank the following people for providing significant inputs to an earlier version of the document that led to this manuscript: Dr Victor Aguayo, Dr France Begin, Dr Rae Galloway, Dr Susan Horton, Ms Nancy Haselow, Dr Lalla Touré, Dr Phil Harvey and Dr Jay Ross.










