The metabolic syndrome (MetS) represents a clustering of central adiposity, dyslipidaemia, hyperglycaemia and elevated blood pressure, being associated with an increased risk of CVD and type 2 diabetes(Reference Hanson, Imperatore and Bennett1–Reference Pyorala, Miettinen and Halonen3). Although its pathogenesis is still incompletely understood, the interaction of obesity, a sedentary lifestyle and dietary, genetic, socio-economic and environmental factors is known to contribute to its development(Reference Ford, Kohl and Mokdad4, Reference Liese, Mayer-Davis and Haffner5).
Increasing evidence indicates that the distribution of MetS varies remarkably among different geographic and socio-economic categories of the population, demonstrating notable health inequalities. Unhealthy behaviours are substantially responsible for epidemic prevalence of diabetes, metabolic disorders and cardiovascular mortality(Reference Carnethon, Loria and Hill6, Reference Yoo, Nicklas and Baranowski7). In contrast, a healthy lifestyle including non-smoking, an appropriate diet, satisfactory physical activity level and a healthy weight can provide substantial cardiovascular and metabolic benefits(Reference Fappa, Yannakoulia and Pitsavos8).
Most of the evidence regarding socio-economic and lifestyle determinants of MetS have come from American(Reference Loucks, Magnusson and Cook9–Reference Zhu, St-Onge and Heshka12) and Asiatic settings(Reference Kim, Kim and Choi13, Reference Xu14) and from southern European countries(Reference Erem, Hacihasanoglu and Deger15, Reference Panagiotakos, Pitsavos and Chrysohoou16). However, these findings were inconsistent owing to diversity in the populations’ characteristics, the criteria used to define MetS, the methodologies of data collection and the selection of confounding factors. Knowledge and understanding of the epidemiological profile of this emerging pathology is an essential prerequisite to assess public health needs and to address population-specific, evidence-based prevention strategies.
Luxembourg is a small country situated in the heart of Europe, hosting foreign residents from over 150 different nationalities, predominantly of European origin. Cardiovascular mortality accounted for about 40 % of total causes of death in 2006(17). A recent study, based on a representative sample of the adult residents in Luxembourg (ORISCAV-LUX), confirmed high prevalences of diabetes, hypertension and lipid disorder, the major components of MetS(Reference Alkerwi, Sauvageot and Donneau18). Additionally, the multiple-risk profile was more prevalent in men than in women, even in younger age groups. These initial findings fostered novel research work to estimate the overall, age- and gender-specific prevalence of MetS and to explore the potential dietary, socio-economic and behavioural factors that may determine the prevalence variations.
The recent guidelines released by the Joint Interim Statement (JIS) 2009 stressed the need to adopt ethnic-specific values of waist circumference (WC) to define MetS. For the Europid population, dual WC references, 94/80 cm v. 102/88 cm for men and women, respectively, were suggested(Reference Alberti, Eckel and Grundy19). The comparison of the various MetS definitions, carried out on European adults of the ORISCAV-LUX study(Reference Alkerwi, Donneau and Sauvageot20), demonstrated an ‘almost perfect’ level of agreement between the JIS (94/80) and the Revised-Adult Treatment Panel (R-ATPIII; 102/88) definitions. Given that the prevalence of MetS varies considerably across ethnic groups(Reference Liu, Grundy and Wang21), the purpose of the present study was to identify the dietary, behavioural and socio-economic determinants of MetS among the adult Europid population residing in Luxembourg using the R-ATPIII criteria.
Methods
Study population
ORISCAV-LUX is a nationwide, cross-sectional survey, conducted between November 2007 and January 2008, to study cardiovascular risk factors among the presumably healthy adult residents of Luxembourg. A representative random sample of 4496 individuals, stratified by sex, age (18–69 years) and district of residence, was selected from the national health insurance registry. Pregnant women (n 21), people living in institutions (n 12), people with serious mental and/or physical handicap (n 5), prisoners (n 1), people outside the determined age range (n 2) and those deceased before recruitment (n 5) were excluded. Overall 3018 individuals did not participate in the study, including invalid addresses (n 213), negative answers (n 502), withdrawal cases (n 80), non-respondents (n 2192) and those could not participate (n 31). Ultimately, a total of 1432 participants completed the recruitment procedure including 1349 (94·2 %) of European origin. The participation rate corresponded to the theoretically expected rate upon which the sample size was calculated(Reference Alkerwi, Sauvageot and Couffignal22). After eliminating missing data in various components of MetS, data from 1319 participants were available for analysis. The predominantly white homogeneous nature of the sample ensured control over the ethnicity factor, hence permitting generalization of the results over the source population. The demographic and socio-economic characteristics of the studied European population were comparable to those of the general ORISCAV-LUX sample. A comprehensive description of the survey design, sampling method and sample representativeness is published elsewhere(Reference Alkerwi, Sauvageot and Donneau18, Reference Alkerwi, Sauvageot and Couffignal22). Briefly, selected individuals were invited through a personal letter followed by a telephone contact to attend study centres. An auto-administered questionnaire covered information on demographic, socio-economic factors (age, gender, country of birth, marital status, income and educational level), physical activity, dietary habits, smoking status, personal medical history, family medical history and current drug therapy. The anthropometric measurements included body height and weight, which were taken on subjects in light clothing without shoes. WC was measured at midway level between the lower rib margin and the iliac crest. Blood pressure was measured at sitting position, after minimum 30 min of rest. Minimum 8-h fasting blood samples were analysed for glucose, HDL cholesterol (HDL-C) and TAG.
All participants were duly informed and consented to take part in the study, which was approved by the National Research Ethics Committee and the National Commission for Private Data Protection.
Definition of metabolic syndrome
The participants had MetS if three or more of the following R-ATPIII criteria(Reference Grundy, Cleeman and Daniels23) were met: (i) raised TAG ≥150 mg/dl or treatment for this lipid anomaly; (ii) reduced HDL-C <40 mg/dl for men or <50 mg/dl for women or treatment for this lipid anomaly; (iii) systolic blood pressure (SBP) ≥130 mmHg or diastolic blood pressure (DBP) ≥85 mmHg or treatment of previously diagnosed hypertension; (iv) fasting plasma glucose (FPG) level ≥100 mg/dl or use of medication for hyperglycaemia; (v) elevated WC ≥102 cm for men or ≥88 cm for women.
Demographic, socio-economic and clinical factors
The education level was classified into three groups: ‘primary’, ‘secondary’ and ‘tertiary’ level. Marital status was recorded as ‘single’, ‘divorced or separated’, ‘widowed’ and ‘married or living with partner’. Work status was classified as ‘employed’ ‘retired’ and ‘unemployed including sick leaver and disabled’. ‘Objective economic status’ was ascertained by asking participants to select the category best representing total monthly household income and to indicate the number of adults and children living in the same household, in order to measure the Adult Equivalent Income (AEI). On the basis of the current official national poverty risk threshold for AEI (National Institute of Statistics), the income variable was classified as either above or below risk of poverty threshold. ‘Subjective economic status’ was assessed by asking ‘To what extent do your current income and other available resources allow you to provide for your needs?’ and was classified as ‘difficult’ or ‘easy’. Family history of diabetes mellitus, arterial hypertension, myocardial infarction and cerebrovascular accident were considered positive when the participant reported that his/her parents or close relatives had history of the selected medical condition.
Lifestyle-related factors
Smoking was categorized as current, former and non-smoker. The current dose-related tobacco exposure was estimated from the number of cigarettes smoked daily, classified as <10, 10–20 and >20 cigarettes/d. Alcohol intake was dichotomised as drinker or non-drinker. Physical activity across a variety of daily situations (leisure time, work, transport and household tasks) was evaluated by means of the International Physical Activity Questionnaire (IPAQ), which categorizes the population into three levels: ‘low’ (physically inactive), ‘moderate’ and ‘high’ levels of physical activity on the basis of scoring criteria.
Dietary factors
Dietary intake data were collected using an FFQ which reports the frequency of consumption and portion size of 134 items over the last 3 months. To describe overall quality diet, a Diet Quality Index (DQI) was developed. It comprised thirteen components assessed on the basis of WHO recommendations for the prevention of chronic diseases(24). The DQI evaluates the intake of key dietary elements that must be supplied sufficiently to guarantee a healthy diet. For each achieved component intake goal, one point was attributed; otherwise a zero was given indicating a poor-quality diet. Components 1–4 were based on SFA, PUFA, MUFA and overall fat consumption as a percentage of total daily energy intake. Component 5 measured the ratio of n-6 to n-3 fatty acids. Component 6 was based on cholesterol intake in mg/d. Components 7–9 were based on total carbohydrates, simple sugars and total protein, respectively, as a percentage of total daily energy intake. Components 10–13 measured Na, fruits/vegetables, and total and soluble fibre intakes in g/d.
Statistical analyses
MetS was considered as the dependent variable, while age, gender, marital status, education level, work status, smoking habits, alcohol consumption, physical activity, dietary habits and family history of selected medical conditions were the independent covariates. ‘Low risk’ participants (younger age, women, non-smokers, non-drinkers, physically active, respect the dietary recommendations, without family history of selected medical conditions) were taken as reference categories. Selection of variables in the multivariate logistic regression analysis was based on a literature review and on statistical criteria (variables showing P < 0·10 were entered in the multivariate model). Non-selected variables were discarded from the final model. The introduction of BMI was tested in another multivariate model.
To account for the stratified random sampling method used to recruit the participants, weighted statistical methods were applied to produce nationally representative estimates. A sampling weight equal to the inverse probability of unit selection was allocated to each participant from the same stratum. This stratum sampling weight was defined as the ratio between the population stratum size and the observed sample stratum size. All statistical analyses were performed using the SAS statistical software package version 9·2 (SAS Institute Inc., Cary, NC, USA). Results were considered to be significant at the 5 % critical level (P < 0·05).
Results
Epidemiology of the metabolic syndrome
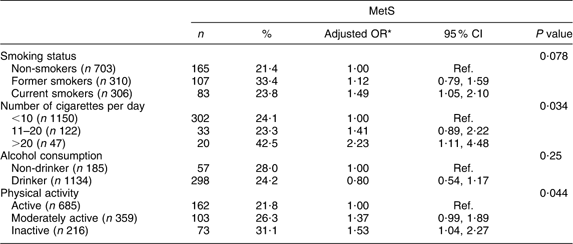
The overall prevalence of MetS among the presumably healthy Europid adults residing in Luxembourg was 18·5 % for women and 30·8 % for men (P < 0·0001). For both genders, the MetS prevalence rate increased remarkably with age, ranging from <1 % among young women to reach a peak of 60 % in elderly men. No interaction with age or with gender was found for MetS (Fig. 1). The salient components in both genders were elevated blood pressure and TAG. The prevalence of each component was significantly higher in men than in women except for abdominal obesity (Fig. 2).
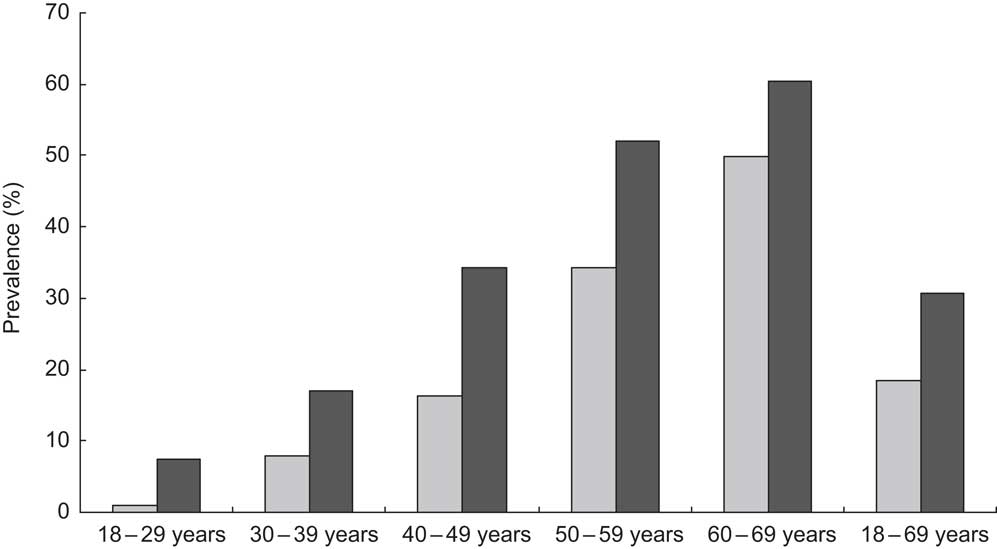
Fig. 1 Gender- and age-specific prevalence of the metabolic syndrome, according to the Revised-Adult Treatment Panel (R-ATPIII) definition, among the European adult residents of Luxembourg, ORISCAV-LUX study (![]() , women;
, women; ![]() , men)
, men)
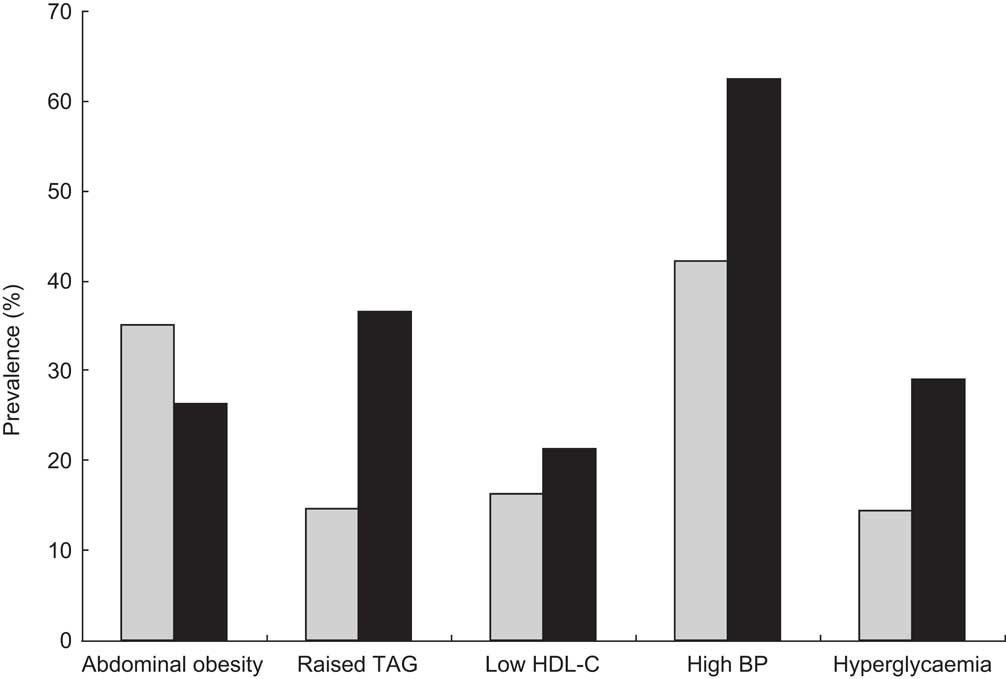
Fig. 2 Prevalence of the metabolic syndrome components (HDL-C, HDL cholesterol; BP, blood pressure), according to the Revised-Adult Treatment Panel (R-ATPIII) definition, among the European adult residents of Luxembourg, ORISCAV-LUX study (![]() , women;
, women; ![]() , men)
, men)
Prevalence of metabolic syndrome and socio-economic factors
Given the marked increase in MetS prevalence with age and significant gender-specific differences, the effect of each socio-economic characteristic was adjusted for these two factors. The possible interaction of each variable with age and gender was also considered. Table 1 displays the age- and gender-adjusted odd ratios for MetS according to socio-economic characteristics. Except for marital status, there were significant associations between the prevalence of MetS and all selected socio-economic characteristics, namely level of education (P < 0·0001), work status (P = 0·037) and subjective (P = 0·024) and objective economic status (P = 0·003). There was a significant work status×age interaction.
Table 1 Prevalence of the metabolic syndrome (MetS) according to socio-economic characteristics among the Europid population of the ORISCAV-LUX study (n 1319)
Ref., referent category.
*OR adjusted for age and gender.
†Only women.
‡Significant interaction with age.
Regardless of age and gender, subjects with primary and secondary level of education were at more than 2-fold and 1·5-fold increased risk of MetS than those with a university degree (OR = 2·34; 95 % CI 1·57, 3·48 and OR = 1·51; 95 % CI 1·04, 2·17), respectively.
The prevalence of MetS was significantly lower in employed participants (19·5 %) than in retired (54·0 %) and non-employed participants, including sick leavers and disabled (37·7 %; P = 0·037), with an interaction effect of age. Considering housewife status as a separate risk factor, non-working women had a significantly higher prevalence of MetS (P < 0·0001) and a twofold increased risk (OR = 2·40; 95 % CI 1·51, 3·80) compared with working women, even after age adjustment.
When considering the objective economic status, MetS was significantly more prevalent in subjects below risk of poverty than in those living above it (P = 0·003). After age and gender adjustment, the former were still at higher risk (OR = 1·75; 95 % CI 1·21, 2·53) compared with the latter. The prevalence of MetS was lower in subjects living easily within their available resources than in those who reported difficult economic living conditions (24·3 % v. 25·9 %, respectively). The age- and gender-adjusted OR among the latter group was 1·56 (95 % CI 1·06, 2·28).
Prevalence of metabolic syndrome and lifestyle factors
The associations between lifestyle factors, such as smoking status, number of cigarettes smoked daily, alcohol consumption and physical activity, and the prevalence of MetS are presented in Table 2. After adjustment for age and gender, current cigarette smokers were at higher risk of developing MetS (OR = 1·49; 95 % CI 1·05, 2·10) than non-smokers. Likewise, smoking >20 cigarettes/d showed a significant positive association with MetS. The OR for MetS in moderately active and inactive subjects compared with active subjects was 1·37 (95 % CI 0·99, 1·89) and 1·53 (95 % CI 1·04, 2·27), respectively. No association was seen between alcohol intake and MetS.
Table 2 Prevalence of the metabolic syndrome (MetS) according to lifestyle characteristics among the Europid population of the ORISCAV-LUX study (n 1319)
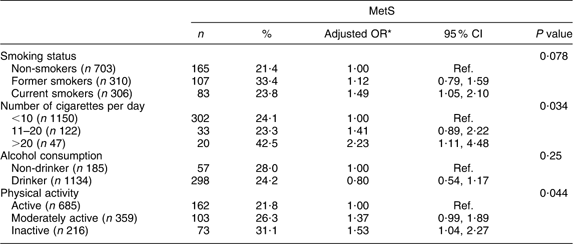
Ref., referent category.
*OR adjusted for age and gender.
Prevalence of metabolic syndrome and family medical history
Only diabetes and arterial hypertension were associated with MetS (OR = 2·40; 95 % CI 1·72, 3·36 and OR = 1·61; 95 % CI 1·17, 2·22, respectively; Table 3).
Table 3 Prevalence of the metabolic syndrome (MetS) according to family history of selected medical conditions among the Europid population of the ORISCAV-LUX study (n 1319)
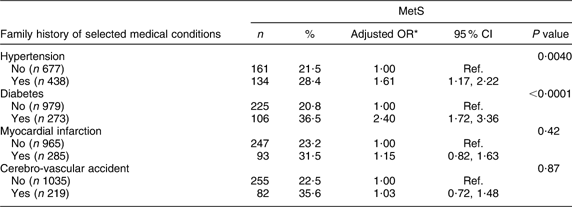
Ref., referent category.
*OR adjusted for age and gender.
Prevalence of metabolic syndrome and dietary factors
A significant association was found between MetS and participants’ adherence to recommendations relative to SFA (P = 0·02), simple sugars (P = 0·0004) and total protein (P = 0·006). Protein consumption lower than 10 % or higher than 15 % of total energy intake was significantly associated with MetS (OR = 1·56; 95 % CI 1·14, 2·15). After age and gender adjustment, no MetS–DQI association was observed (Table 4).
Table 4 Prevalence of the metabolic syndrome (MetS) according to compliance to key dietary recommendations for different food components among the Europid population of the ORISCAV-LUX study (n 1229)
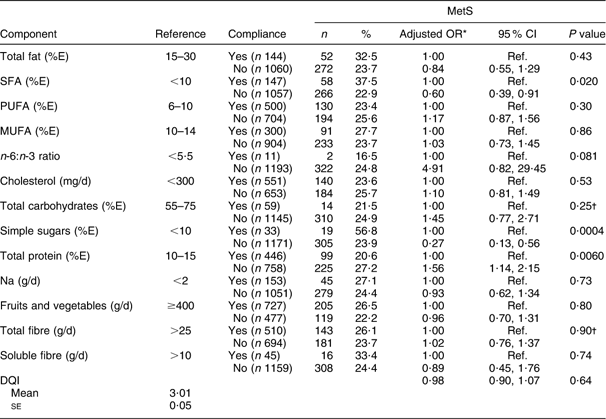
%E, percentage of total daily energy intake; DQI, Diet Quality Index.
*OR adjusted for age and gender.
†Interaction with age is presented.
Metabolic syndrome modelling
The final model of MetS with respect to the covariates studied demonstrated that age, male gender, primary level of education, physical inactivity, family history of diabetes and hypertension and inadequate total protein intake were significant determinants of MetS after adjustment for all other factors (Table 5). A sensitivity approach was applied to this final model by testing the significance effect of the rejected variables one by one. No additional variable was found to contribute significantly to the model. It is worth mentioning that by adding BMI to the other significant socio-economic, dietary and behavioral factors in the model, only age, gender, family history of diabetes and BMI remained significantly associated with MetS (data not shown).
Table 5 Final multivariate logistic regression model associating prevalence of the metabolic syndrome (MetS) with demographic, socio-economic, behavioural and dietary characteristics of the Europid population of the ORISCAV-LUX study
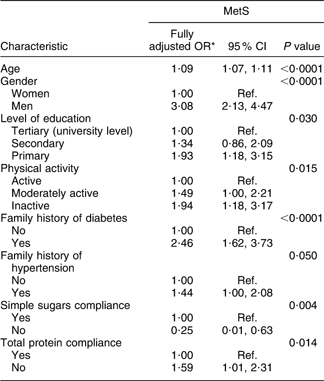
Ref., referent category.
*OR adjusted for other socio-economic, family history, dietary and lifestyle factors.
Discussion
The innovative purpose of the present study was to investigate, for the first time, the epidemiology of MetS in the European population residing in Luxembourg and to discern the potential socio-economic, dietary and behavioural factors that could explain its variability, in order to suggest prevention strategies addressing the real needs of the groups at risk.
The emerging data from European studies suggest that MetS is a common cardio-metabolic disorder, with a prevalence rate ranging from a low 18 % in Italy(Reference Mannucci, Monami and Bardini25) to a high 38 % in Turkey(Reference Can and Bersot26). Nearly 25 % of the Europids in Luxembourg are affected by MetS with significant gender difference. Consistent with previous studies(Reference Hildrum, Mykletun and Hole27), the MetS prevalence rate increased dramatically with age, in both genders. This effect can be largely explained by age-related rises of blood pressure and glucose level(Reference Alexander, Landsman and Grundy28). Globally this prevalence rate is comparable to worldwide estimates, although lower than in the USA (34·5 %)(Reference Ford and Giles29) and higher than in Australia (15 %)(Reference Adams, Appleton and Wilson30) or China (17 %)(Reference Liu, Grundy and Wang21).
The prevalence of MetS was significantly higher in our participants with a family history of diabetes and hypertension, regardless of age and gender, and after adjustment for other potential socio-economic, dietary and behavioural factors; hence, this particular subgroup of the population is more susceptible to develop MetS, probably due to genetic predisposition to components of MetS(Reference Erem, Hacihasanoglu and Deger15).
The prevalence of MetS also differed significantly according to subjective economic status and objective household income independently of age and gender; however, these associations became non-significant in multivariate analyses. An independent MetS–household income association was observed in the USA(Reference Loucks, Magnusson and Cook9, Reference Park, Zhu and Palaniappan31), and in French(Reference Dallongeville, Cottel and Ferrieres32) and in Korean women only(Reference Park, Yun and Lee33). Marital status had no effect on MetS prevalence when adjusting for age and gender in the present study. By contrast, MetS prevalence differed significantly according to work status; sick leavers, disabled and retired participants were more likely to be affected by MetS than the employed group, irrespective of age and gender. Interestingly, housewives were also more at risk of MetS at any age.
One of the salient findings of the present study concerns the inverse and independent association of education level with MetS. Adjusting for dietary, behavioural and other socio-economic factors did not narrow the differences, suggesting a clear educational disparity in the prevalence of MetS among European adults in Luxembourg. This finding is consistent with previous studies(Reference Lidfeldt, Nyberg and Nerbrand34, Reference Goyal, Bhatt and Steg35) that reported pronounced inverse associations between educational level and features of MetS, particularly in women(Reference Loucks, Magnusson and Cook9, Reference Wamala, Lynch and Horsten36). Better education definitely facilitates the understanding and acquisition of a healthy lifestyle and hence greater protection against MetS. Moreover, highly educated individuals enjoy better social, psychological and economic support(Reference Winkleby, Jatulis and Frank37). In a wealthy country like Luxembourg, higher education generally leads to a better income and standard of life. Lower education and limited financial resources jointly lead people to select low-cost, unhealthy, energy-dense foods composed of fat, refined grains and added sugar. This type of food favours the development of insulin resistance, hypertriacylglycerolaemia and weight gain(Reference Dallongeville, Cottel and Ferrieres32, Reference Drewnowski and Specter38). Another frequently cited correlate with low socio-economic status is the stress that might induce a psychological defeat reaction, which in turn would activate the hypothalamic–pituitary–adrenal axis and trigger cardiovascular risk(Reference Park, Yun and Lee33). The psychosocial stress may result from the challenges of daily life, a poor social network and/or uncertainties about future prospects related to social or material disadvantage(Reference Gallo and Matthews39). As a vicious cycle, people in the lowest income categories are most often unemployed and/or unqualified and are bothered by their limited resources, resulting in reduced physical activity and/or stress(Reference Dallongeville, Cottel and Ferrieres32). This in turn favours weight gain and insulin resistance, major precursors of MetS. In spite of the cross-sectional nature of the findings obtained, the negative association between education level and MetS is understandable and the hypothesis of reverse causality is not likely to be addressed.
Smoking is known to be highly related to cardiovascular risk and appears to have an adverse effect on several components of MetS, such as hyperglycaemia and high blood pressure(Reference Park, Zhu and Palaniappan31, Reference Dzien, Dzien-Bischinger and Hoppichler40, Reference Retnakaran, Hanley and Connelly41). In the present study, a positive dose–response relationship between smoking exposure (>20 cigarettes/d) and MetS was found irrespective of age and gender; however, this association disappeared when adjusting for other confounders. A positive and dose-dependent relationship between the number of cigarettes smoked daily and MetS was found in previous studies(Reference Masulli and Vaccaro42, Reference Oh, Yoon and Lee43). The exact mechanism of how cigarette smoking increases the risk of MetS remains uncertain, although pharmacological actions of cigarette smoking and nicotine on lipoprotein metabolism and insulin resistance have been demonstrated(Reference Facchini, Hollenbeck and Jeppesen44, Reference Nakanishi, Takatorige and Suzuki45).
Regarding alcohol consumption, the literature shows inconsistent findings owing to the complex mechanistic relationship with each component of MetS. Mild to moderate alcohol use has a favourable influence on lipid metabolism, abdominal obesity and glucose regulation(Reference Koppes, Dekker and Hendriks46). In contrast, excessive alcohol consumption causes hypertension(Reference Xin, He and Frontini47) and hypertriacylglycerolaemia(Reference Kato, Kiyohara and Kubo48). A recent meta-analysis suggested that ‘responsible moderate drinking’ places the individual at lower risk of having MetS(Reference Alkerwi, Boutsen and Vaillant49). No significant association was seen in the present ORISCAV-LUX study, although this finding should be interpreted with caution: the alcohol consumption data were based on self-declaration with the possibility of misclassification of exposure due to under-reporting; additionally, in such a population-based study aiming to observe cardiovascular risk factors, ‘social desirability’ might push participants to under-report their actual alcohol consumption level. The overall self-reported alcohol drinking during the last 12 months (5·4 g/d, i.e. half a standard glass/d) was sensibly under-estimated.
The prevalence of MetS varied significantly according to the level of physical activity, showing an independent association after full multivariate adjustment for other factors. Only highly active subjects seemed predominantly protected, suggesting the need to recommend a more active lifestyle for those prone to MetS. The self-reported measures may overestimate the actual physical activity and result in underestimation of the association between moderate activity and the disease outcome(Reference Rennie, McCarthy and Yazdgerdi50). In our study, bias related to self-misclassification cannot be excluded and may explain the attenuated moderate activity–MetS association. In spite of methodological shortcomings, self-reported measures remain the most feasible and affordable instruments for global surveillance of physical activity. Another advantage of the IPAQ is that physical activity is expressed in MET-min/week (MET, metabolic equivalent task) rather than energy expenditure (kJ/kcal), resulting in physical activity estimates independently of body weight. Although comparison between studies is difficult owing to different MetS definitions and dissimilar physical activity evaluation tools, our findings are in agreement with previous studies which showed an inverse MetS–physical activity association(Reference Ford, Kohl and Mokdad4, Reference Irwin, Ainsworth and Mayer-Davis51, Reference Rennie, Hemingway and Kumari52). To the best of our knowledge, the present study is the first population-based study that has examined the association between MetS and physical activities according to IPAQ. Another study, based on the Minnesota leisure-time physical activity questionnaire, documented that moderate and vigorous physical activity were both associated with a reduced risk of MetS, independently of age, smoking and high alcohol intake(Reference Rennie, McCarthy and Yazdgerdi50). In a study on young adults, sedentary behaviours measured by the number of hours of watching television or playing video/computer games were positively associated with the presence of at least three metabolic risk factors(Reference Gustat, Srinivasan and Elkasabany53). While physical activity was significantly associated with high HDL-C level(Reference Pitsavos, Panagiotakos and Weinem54), the adverse effect of inactivity, attributable to reduced energy expenditure, was inversely associated with body weight, blood pressure and TAG(Reference Ford, Kohl and Mokdad4).
In the present study, the association of MetS with key individual dietary components was investigated. In addition, a composite measure of diet quality (DQI) was created to evaluate the overall healthfulness of dietary intake, in terms of adherence to the dietary guidelines. Unexpectedly, participants who were non-compliant with the simple sugars and SFA guidelines showed significantly lower odds of having MetS, regardless of their age and gender. The confirmed association between simple sugars and MetS in the fully adjusted model may be explained by the reverse causality inherent to the cross-sectional nature of the study. It seems that suffering from dyslipidaemia or diabetes may significantly encourage the participants to limit their intake of SFA or simple sugars. In addition, the limited number of subjects in the compliant category (thirty-three cases, 2·7 %) may underpowered to infer about the harmful effect of simple sugars on MetS. An exploratory analysis showed that participants with diabetes and lipid disorders did better in adhering to the recommendations than those free of these pathologies. Moreover, a significant proportion of participants having MetS were currently on one or more diets, for example to lower their cholesterol, sugar intake, blood pressure or lose weight (data not shown).
Another striking result of the ORISCAV-LUX study was the persistent independent association between MetS and total protein intake, even after adjustment for age, gender, education, socio-economic factors, smoking, physical activity, family medical history and other dietary factors (including SFA). These findings are, at least in part, in agreement with the prospective Japanese–Brazilian Diabetes Study, in which specifically red meat consumption (major source of protein and fat) was associated with an almost fivefold increase in the risk of MetS in men(Reference Damiao, Castro and Cardoso55). This nutrient-specific observation requires further in-depth investigation regarding the type of protein (for example, animal red meats) and the potential confounding role of total fat and its components (SFA, MUFA and PUFA). Considering the DQI, no association was seen with MetS after adjusting for potential confounding factors, suggesting that the overall quality of diet had no significant impact on the prevalence of MetS in our studied population.
When considering BMI among other potential confounders in the final model (data not shown), only age, gender and family history of diabetes remained independently associated with the high prevalence of MetS, suggesting that BMI is the most sensitive marker among associated factors for MetS. This finding is consistent with results from US and Korean adults, in which the BMI was shown to be a strong predictor for MetS(Reference Park, Zhu and Palaniappan31, Reference Park, Oh and Cho56).
The present study is characterized by several strong points. The ORISCAV-LUX is based on a recent nationwide sample of Luxembourg adult residents(Reference Alkerwi, Sauvageot and Donneau18). A detailed study of non-participants showed comparable demographic and clinical characteristics of participants and non-participants, hence providing population-representative prevalence estimates(Reference Alkerwi, Sauvageot and Couffignal22). Nevertheless, a number of limitations should be recognized. First, the cross-sectional design has inherent drawbacks and precludes causal inferences, notably regarding the MetS–simple sugars association. Second, the analyses were based on the Europid population; our results may therefore not apply to other ethnic groups. Third, as in most epidemiological studies, the risk exists for incomplete adjustment for latent confounders, such as psychosocial factors. Finally, evaluation of tobacco consumption, alcohol use, physical activity and food habits was based on self-reported information. Although the participants completed the auto-administered questionnaire with the help and supervision of well-trained staff, the possibility of under- or over-reporting cannot be excluded. This might lead to a selective misclassification of high-risk individuals which could blur the potential association.
In summary, from a public health standpoint, our findings suggest that male gender, age, low education, family history of diabetes and hypertension as well as specific behavioural and lifestyle factors, such as physical inactivity and inadequate protein intake, account for the disparities observed in MetS prevalence rates among the studied population. The multiplicity of factors contributing to MetS highlights the potential interactions that occur at both individual and behavioural levels to produce the condition. MetS does not develop overnight, but rather becomes established over years of overlapping dietary, behavioural and inherited risk factors. These findings support the need for a comprehensive approach in clinical and community settings to promote healthier habits.
Acknowledgements
The work was supported by a research grant from the National Fund of Research (Fond National de Recherche; project MSF, 784844, BM). The authors declare that they have no competing interest. A. Alkerwi was involved in the conception and design of the ORISCAV-LUX survey, coordinated the field data collection, conceived the present research, contributed to data analyses and drafted the manuscript. A.-F.D. conducted the statistical analyses and discussion of the results. N.S. participated in the statistical analyses. A. Albert provided critical reviews of the manuscript and intellectual content. M.G. provided expertise and oversight throughout the process. All authors reviewed drafts and approved the final version of the manuscript. The ORISCAV-LUX study was made possible by the people who agreed to participate and the financial support of government (Ministry of Health and Ministry of Research).