INTRODUCTION
Very preterm (<32 weeks gestational age; VP) and very low birth weight (<1500 g, VLBW) infants grow up with life-long risks for neurocognitive impairment (Breeman, Jaekel, Baumann, Bartmann, & Wolke, Reference Breeman, Jaekel, Baumann, Bartmann and Wolke2015; Eryigit Madzwamuse, Baumann, Jaekel, Bartmann, & Wolke, Reference Eryigit Madzwamuse, Baumann, Jaekel, Bartmann and Wolke2015). Early identification allows timely provision of resources. Follow-up after VP/VLBW birth is a general standard in many Western countries (Doyle et al., Reference Doyle, Anderson, Battin, Bowen, Brown, Callanan and Woodward2014). However, assessing cognitive development through descriptive behavioral data is time consuming and expensive (Aslin & Fiser, Reference Aslin and Fiser2005; Hack et al., Reference Hack, Taylor, Drotar, Schluchter, Cartar, Wilson-Costello and Morrow2005). Repeated head circumference assessments may be a cost-effective indicator of brain development (Bartholomeusz, Courchesne, & Karns, Reference Bartholomeusz, Courchesne and Karns2002; Cheong et al., Reference Cheong, Hunt, Anderson, Howard, Thompson, Wang and Doyle2008; Garcia-Alix, Saenz-de Pipaon, Martinez, Salas-Hernandez, & Quero, Reference Garcia-Alix, Saenz-de Pipaon, Martinez, Salas-Hernandez and Quero2004). Regional brain and cortical growth is significantly associated with brain maturation (Makropoulos et al., Reference Makropoulos, Aljabar, Wright, Hüning, Merchant, Arichi and Rueckert2016), generally indicating that size matters.
Head circumference is measured in infancy in most countries, but its predictive validity remains controversial (Wright & Emond, Reference Wright and Emond2015). At term, preterm infants have smaller whole brain volumes than term born infants (Ball et al., Reference Ball, Boardman, Rueckert, Aljabar, Arichi, Merchant and Counsell2012; Makropoulos et al., Reference Makropoulos, Aljabar, Wright, Hüning, Merchant, Arichi and Rueckert2016). Researchers argue that head growth between birth and 2 years is critical for intellectual development (Räikkönen et al., Reference Räikkönen, Forsén, Henriksson, Kajantie, Heinonen, Pesonen and … Eriksson2009) and studies of preterm populations have documented the value of head growth as a predictor of long-term neurocognitive abilities (Sammallahti et al., Reference Sammallahti, Heinonen, Andersson, Lahti, Pirkola, Lahti and … Raikkonen2017, Reference Sammallahti, Pyhala, Lahti, Lahti, Pesonen, Heinonen and … Raikkonen2014). Accelerated postnatal head growth suggests catch up after prenatal restraint (Cockerill, Uthaya, Doré, & Modi, Reference Cockerill, Uthaya, Doré and Modi2006), but may not compensate for poor earlier growth after infancy (Gale, O’Callaghan, Bredow, & Martyn, Reference Gale, O’Callaghan, Bredow and Martyn2006). Thus, it is important to investigate whether there are certain time windows of head growth that matter most for later IQ development.
In clinical practice, individual head growth is usually documented by plotting raw head circumference values on age-standardized growth chart curves, by converting raw values into standard scores, or by categorizing scores (Fenton & Kim, Reference Fenton and Kim2013). This is done to (clinically) identify individuals who grow at a substantially slower than average rate. Instead, growth over time may be assessed with latent class growth curve analyses to identify how the growth rate across all individuals (i.e., head growth) affects growth in another dimension (i.e., IQ). Structural equation modeling (SEM) has been proposed as the most appropriate approach in the context of additional factors (e.g., gestational age, birth weight) (Usami, Hayes, & McArdle, Reference Usami, Hayes and McArdle2017).
Our aims were (i) to investigate VP/VLBW and term born individuals’ head growth from birth to 4 years and intelligence in childhood and adulthood, and (ii) to determine the specific timing of head growth that matters for intelligence development.
METHODS
Design
Participants were all VP and/or VLBW infants and an equally sized group of healthy term comparisons born in a geographically defined area of South Bavaria (Germany) between January 1985 and March 1986 as part of the prospective whole population Bavarian Longitudinal Study (BLS). The current study uses data collected at birth; at 5 and 20 months; and at 4, 6, 8, and 26 years of age.
Standard protocol approvals and patient consents
This research was completed in accordance with the Helsinki Declaration. Original ethical approval was obtained from the University of Munich Children’s Hospital and the Landesärztekammer Bayern. Ethical approval for the adult follow-up was granted by the Ethical Board of the University Hospital Bonn (reference 159/09). Informed written consent was provided by parents within 48 hr of their child’s birth and all participants gave fully informed written consent for the adult assessment.
Participants
This study assesses a whole population sample of 682 VP/VLBW individuals (Wolke & Meyer, Reference Wolke and Meyer1999). Of this cohort, 411 VP/VLBW were presumed alive, living in Germany, and eligible for inclusion at 26 years of age, and 260 (63.3%) participated in the adult assessment (see Appendix Figure 1). The BLS VP/VLBW participants did not differ from VP/VLBW adults who dropped out in terms of GA, BW, duration of hospitalization, gender, maternal age, parental marital status, and childhood cognitive scores, but had fewer prenatal complications and were of higher socioeconomic status (SES) (Eryigit Madzwamuse et al., Reference Eryigit Madzwamuse, Baumann, Jaekel, Bartmann and Wolke2015).
Of 916 healthy term-born comparison infants at birth, 350 were selected and stratified to match the VP/VLBW participants at the 6 years follow-up assessment. Of these, 308 individuals were eligible for inclusion and 229 (74.4%) participated at 26 years (Eryigit Madzwamuse et al., Reference Eryigit Madzwamuse, Baumann, Jaekel, Bartmann and Wolke2015). Only participants who had complete childhood and adulthood IQ data (203 VP/VLBW and 198 term comparisons) were included in the current analyses.
Head circumference
Head circumference (in centimeters) was assessed by trained research nurses during clinical assessments at birth; 5 and 20 months; and at 4, 6, and 8 years of age. At 5 and 20 months, examination ages were corrected for gestational age at birth. HC was measured twice at each assessment and the average score was recorded. Raw HC scores and standardized scores are reported in Table 1. Raw scores are used in main analyses to investigate separate contributions of gestation and birth weight in our statistical models.
Table 1 Descriptive characteristics of the VP/VLBW and term participants from birth to age 26 years

Note. Data are presented as mean (standard deviation) if not indicated otherwise; all assessments at 5 and 20 months were adjusted for gestational age at birth.
a χ²-Value.
b Standardized according to current international World Health organization (WHO) child growth standard scores (WHO, 2007).
CI=confidence interval; DQ=developmental quotient; IQ=intelligence quotient; SES=socioeconomic status; VLBW=very low birth weight; VP=very preterm.
Biological and demographic characteristics
Gestational age (weeks, range: VP/VLBW=25–36, term comparisons=37–42) and birth weight (grams, range: VP/VLBW=630–2590, term comparisons=2120–5050) were coded from Bavarian perinatal survey forms at birth (Zander, Holzmann, & Selbmann, Reference Zander, Holzmann and Selbmann1989). Family SES at birth was assessed as a weighted composite score of parents’ education and occupation (Bauer, Reference Bauer1988).
Neurocognitive assessments
Cognitive development (i.e., intelligence) was assessed longitudinally by psychologists with standardized tests in childhood and adulthood (Breeman et al., Reference Breeman, Jaekel, Baumann, Bartmann and Wolke2015). At age 20 months, children were administered the Griffiths’ Mental Development Scales Developmental Quotient (DQ) items (Brandt, Reference Brandt1983). At 6 and 8 years, IQ was assessed with the German version of the Kaufman Assessment Battery for Children (K-ABC) (Melchers & Preuss, Reference Melchers and Preuss1991). Reliability and construct validity of the K-ABC are high [e.g., 0.70 correlation with the Wechsler Intelligence Scale for Children-Revised (WISC-R) total score] (Melchers & Preuss, Reference Melchers and Preuss1991). At 26 years, age-normed Full-Scale IQ was assessed with a short German version of the Wechsler Adult Intelligence Scale (WAIS III) (Von Aster, Neubauer, & Horn, Reference Von Aster, Neubauer and Horn2006). In our models, VP/VLBW individuals’ DQ and IQ data from different ages are directly compared to healthy term born comparison individuals’ DQs and IQs at each assessment point.
Statistical approach
Data were analyzed with SPSS 23 and Amos 24 (Armonk, NY: IBM Corp.). Structural Equation Modeling (SEM) was used to simultaneously test direct and indirect associations between head circumference and growth, and intelligence to 26 years of age. Gestational age, birth weight, and family SES were included as additional predictors of interest. In the initial, full model, all variables and the potential paths between each measure were included (see Appendix Figure 2). Model fit values were then used to identify the best fitting and most parsimonious final model, indicating the timing of head growth that matters most for intelligence development. Final model results are presented combined for VP/VLBW and term comparisons (Model 1), and as separate multiple-group models (Models 2 and 3).
RESULTS
On average, VP/VLBW had lower head circumference at birth, lower head growth from 20 months to 4 years, and lower intelligence scores than term born comparison individuals (Table 1). However, VP/VLBW had higher head growth from birth to 5 months and, on average, from birth to 20 months than term born comparison infants, both assessments were scheduled corrected for gestational age.
The initial, full model did not have sufficient fit [χ 2=80.44(df=16); p<.001; comparative fit index (CFI)=.983; root mean square error of approximation (RMSEA)=1.00 (90% confidence interval (CI) [.079 to .123]; p of close fit (PCLOSE)=.000; Appendix Figure 2] and was thus reduced to the best fitting, most parsimonious Model 1 (Figure 1). In particular, there was no added value in assessing head growth from birth to 5 months and then from 5 to 20 months separately; thus, growth from birth to 20 months was collapsed into one variable. Moreover, cognitive development at 20 months was less accurately predicted by infants’ head growth than IQ at 6+ years.

Fig. 1 Final structural equation Model 1. Neurocognitive cascades of head growth and intelligence from birth to adulthood in VP/VLBW and term born individuals (n=401).
Model 1 showed that head circumference at birth and head growth in childhood predicted intelligence development from age 6 to 26 years in both VP/VLBW and term born individuals. Effects of gestation and birth weight on intelligence were found to be fully mediated by head circumference and growth (i.e., indirect effects) while family SES directly predicted intelligence. Table 2 shows detailed direct, indirect, and total effects for all variables in the model. The total variance explained (R 2 ) in intelligence was 29% at 6 years, 73% at 8 years, and 70% at 26 years of age. Overall model fit was excellent with χ 2 =19.85(df=16); p<.227; CFI=.999; RMSEA=.025 (90% CI [.000–.055]; PCLOSE=.907.
Table 2 Standardized direct, indirect, and total effects (i.e., path coefficients) of the predictors in the final overall Model 1 (N=401)

Note. HC=head circumference, HG=head growth, IQ=intelligence quotient; SES=socioeconomic status.
Since part of the explained variance in adult IQ was carried by childhood IQ (i.e., longitudinal stability of IQ), we also explored how much variance was explained if only the isolated effects of HC at birth and head growth were used to predict adult IQ. A substantial R 2 =.24 were explained just by HC at birth and early head growth in the total sample (R 2 =.22 in VP/VLBW and R 2 =.02 in healthy term control individuals, respectively).
To test if the associations between head growth and intelligence were different among VP/VLBW compared with term comparison individuals, we ran the same SEM separately as a multiple-group model. As before, head circumference at birth, head growth from birth to 20 months, and from 20 months to 4 years predicted intelligence development from age 6 to 26 years in VP/VLBW and term born individuals, but the path coefficients were slightly different (Figures 2 and 3).

Fig. 2 Multigroup structural equation Model 2. Neurocognitive cascades of head growth and intelligence from birth to adulthood in VP/VLBW individuals only (n=203). Please note: Solid lines represent significant paths, dotted lines represent non-significant paths that were freely estimated in the model
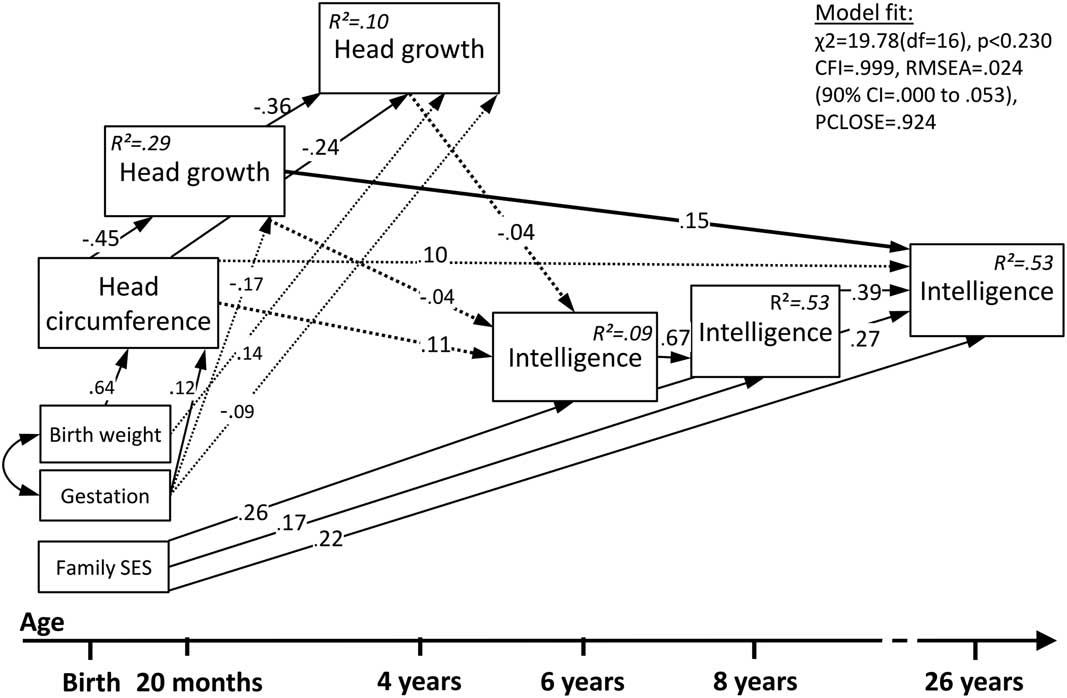
Fig. 3 Multigroup structural equation Model 3. Neurocognitive cascades of head growth and intelligence from birth to adulthood in term born individuals only (n=198). Please note: Solid lines represent significant paths, dotted lines represent non-significant paths that were freely estimated in the model
Model 2 shows that among VP/VLBW, head circumference at birth and head growth in early childhood directly predicted intelligence at age 6, while there were only indirect effects on intelligence at 8 and 26 years. The total variance explained in intelligence was 22% at 6 years, 79% at 8 years, and 73% at 26 years.
Model 3 shows that among term comparisons, only head growth from birth to 20 months directly predicted intelligence at age 26. The total variance explained in term comparison individuals’ intelligence was lower with 9% at 6 years, 53% at 8 years, and 53% at 26 years.
As before, model fit was excellent with χ 2 =19.78(df=16); p<.230; CFI=.999; RMSEA=.024 (90% CI [.000–.053]; PCLOSE=.924; and effects of gestation and birth weight on intelligence were fully mediated by head circumference and growth while family SES directly predicted intelligence.
DISCUSSION
This longitudinal investigation from birth to adulthood confirms that early head growth as a proxy of brain development predicts later intelligence in VP/VLBW but also in term born individuals. Larger head circumference at birth and higher head growth during the first 4 years predicted IQ from age 6 to 26 years in both VP/VLBW and term born individuals while effects of gestation and birth weight on intelligence were fully mediated by head circumference and growth. These findings add to previous evidence about the value of repeated head circumference assessments in early childhood, in particular in VP/VLBW children, as a proxy of brain volume and screening for later intellectual impairments (Kapellou et al., Reference Kapellou, Counsell, Kennea, Dyet, Saeed, Stark and Edwards2006).
Head growth is driven by brain growth and thus an indicator of brain development (Kiesler & Ricer, Reference Kiesler and Ricer2003). Researchers have suggested that the same genetic factors have an effect on physical (i.e., head) growth and cognitive development (Silventoinen, Iacono, Krueger, & McGue, Reference Silventoinen, Iacono, Krueger and McGue2012). However, postnatal brain growth is the product of complex mechanisms, including gray matter maturation (i.e., axon and dendrite sprouting, synapse formation, cortical gyrification) and white matter formation (i.e., glial cell proliferation, myelination). Anatomically, the cranium surrounds and protects the brain and brainstem, and two thirds of its growth occur by 2 years of age (Kiesler & Ricer, Reference Kiesler and Ricer2003).
In healthy term born infants, cortical thickness reaches 97% of adult size by age two (Lyall et al., Reference Lyall, Shi, Geng, Woolson, Li, Wang and Gilmore2015) and cortical gyrification also happens during the first 2 years (Li et al., Reference Li, Wang, Shi, Lyall, Lin, Gilmore and Shen2014), whereas cortical surface area expansion mainly drives brain growth after 2 years of age (Lyall et al., Reference Lyall, Shi, Geng, Woolson, Li, Wang and Gilmore2015). Lower gestation at birth is associated with smaller whole brain volume and growth at term-equivalent age (Kidokoro et al., Reference Kidokoro, Anderson, Doyle, Woodward, Neil and Inder2014; Padilla, Alexandrou, Blennow, Lagercrantz, & Ådén, Reference Padilla, Alexandrou, Blennow, Lagercrantz and Ådén2015) and age 3 months (Holland et al., Reference Holland, Chang, Ernst, Curran, Buchthal, Alicata and Dale2014). Additionally, premature birth is associated with regional brain alterations, particularly in subcortical structures such as the thalamus and striatum as well in lateral temporal and parietal cortices (Ball et al., Reference Ball, Boardman, Rueckert, Aljabar, Arichi, Merchant and Counsell2012; Meng et al., Reference Meng, Bauml, Daamen, Jaekel, Neitzel, Scheef and Sorg2016). These alterations persist into adulthood and are not only accompanied by widespread changes in cerebral white matter (Bäuml et al., Reference Bäuml, Daamen, Meng, Neitzel, Scheef, Jaekel and Sorg2015; Meng et al., Reference Meng, Bauml, Daamen, Jaekel, Neitzel, Scheef and Sorg2016) but also associated with impairments in neurocognitive and behavioral development (Kidokoro et al., Reference Kidokoro, Anderson, Doyle, Woodward, Neil and Inder2014; Parker et al., Reference Parker, Mitchell, Kalpakidou, Walshe, Jung, Nosarti and … Allin2008).
Head circumference is an excellent indicator of cerebral volume measured with magnetic resonance imaging (MRI) in 1- to 6-year-old children (r=0.59–0.93), but its predictive validity is weaker in typically developing older children, adolescents, and adults (r=0.45 to 0.69) (Bartholomeusz et al., Reference Bartholomeusz, Courchesne and Karns2002; Lange, Froimowitz, Bigler, & Lainhart, Reference Lange, Froimowitz, Bigler and Lainhart2010). Accordingly, head circumference may present an easily assessable proxy of overall brain volume, but only during the first years of life, and prediction from HC measurement alone is similar to that of term MRI (Anderson et al., Reference Anderson, Treyvaud, Neil, Cheong, Hunt, Thompson and Inder2017).
With regard to the timing of head growth, SEM indicated that the period from birth to 20 months of age mattered most for directly predicting later IQ development, but indirect effects of head growths from 20 months to 4 years of age were also relevant. This suggests that accelerated brain growth during the first 20 months after preterm birth may be most beneficial for age-appropriate IQ development later in life, in particular for infants born VP/VLBW. Moreover, and in accordance with studies of normative brain growth (Li et al., Reference Li, Wang, Shi, Lyall, Lin, Gilmore and Shen2014; Lyall et al., Reference Lyall, Shi, Geng, Woolson, Li, Wang and Gilmore2015), the significant direct effect of head growth from birth to 20 months on term adults’ IQs suggests that the first 2 years of postnatal life may represent a critical window for healthy brain growth and development. Thus, regular head circumference measurements, used in conjunction with other neurodevelopmental tools, may be recommended until at least 2 years of age to screen for potential brain growth delay. As a result, clinicians may need additional training to correctly administer this effective and inexpensive screening tool (James, Perszyk, MacGregor, & Aldana, Reference James, Perszyk, MacGregor and Aldana2015).
Children’s IQ development is influenced by genetic (Marioni et al., Reference Marioni, Davies, Hayward, Liewald, Kerr, Campbell and Deary2014) and environmental factors, such as parents’ socioeconomic status (Sameroff, Seifer, Barocas, Zax, & Greenspan, Reference Sameroff, Seifer, Barocas, Zax and Greenspan1987), children’s home literacy environments (Jaekel, Schölmerich, Kassis, & Leyendecker, Reference Jaekel, Schölmerich, Kassis and Leyendecker2011), and preschool quality (Melhuish, Reference Melhuish2011). Accordingly, environmental stimulation may promote cognitive development of both VP/VLBW and full term children (Wolke, Jaekel, Hall, & Baumann, Reference Wolke, Jaekel, Hall and Baumann2013); thus, early identification of those children who are at-risk for low IQ would allow timely provision of resources and intervention.
Our observational study results substantiate the validity of studies that aim to improve preterm infants’ long-term cognitive outcomes by supporting early head growth. Such strategies may target individualized neonatal nutrition, adaptive feeding, and breastfeeding to stimulate growth and neurodevelopmental outcomes (Belfort et al., Reference Belfort, Anderson, Nowak, Lee, Molesworth, Thompson and Inder2016; Christmann et al., Reference Christmann, Roeleveld, Visser, Janssen, Reuser, van Goudoever and van Heijst2017). Recently, the microbiome-gut-brain axis has also received attention due to its potential for neuroprotection against white matter injury (Keunen, van Elburg, van Bel, & Benders, Reference Keunen, van Elburg, van Bel and Benders2015). Other avenues toward intervention may target “nutrition for the brain” via environmental stimulation [e.g., the neonatal intensive care unit environment (Pineda et al., Reference Pineda, Neil, Dierker, Smyser, Wallendorf, Kidokoro and … Inder2014), sensitive parenting (Milgrom et al., Reference Milgrom, Newnham, Anderson, Doyle, Gemmill, Lee and … Inder2010; Wolke et al., Reference Wolke, Jaekel, Hall and Baumann2013), preschool education (McCormick et al., Reference McCormick, Brooks-Gunn, Buka, Goldman, Yu, Salganik and Casey2006), and family psychosocial support (Benzies, Magill-Evans, Hayden, & Ballantyne, Reference Benzies, Magill-Evans, Hayden and Ballantyne2013)].
Our sample represents one of the largest whole-population longitudinal studies of neurocognitive development after VP/VLBW birth. In total, 68% of the eligible VP/VLBW and term comparisons recruited at birth were assessed at 26 years; however, the dropout was not random, as low SES families were less likely to continue participation. Social factors are a major reason for dropout in most longitudinal studies (Hille, Elbertse, Gravenhorst, Brand, & Verloove-Vanhorick, Reference Hille, Elbertse, Gravenhorst, Brand and Verloove-Vanhorick2005), and analyses were controlled for SES at birth. Participants were born in Germany in 1985/1986, before the introduction of pioneering new treatments such as surfactant administration, which significantly improved high risk infants’ survival rates. However, studies have shown that increased survival may not result in equivalent improvement of long-term neurocognitive outcomes (Cheong et al., Reference Cheong, Anderson, Burnett, Roberts, Davis, Hickey and Doyle2017; Moore et al., Reference Moore, Hennessy, Myles, Johnson, Draper, Costeloe and Marlow2012; Wolke et al., Reference Wolke, Strauss, Johnson, Gilmore, Marlow and Jaekel2015); thus, the current findings may be as relevant to infants born today as to those born 30 years ago.
Changes of absolute measurements of head circumference according to postnatal age (adjusted for gestation at birth at the 5 and 20 months assessments) were included in our models. From a clinical perspective, it might be preferable to use standardized Z-scores; however, our aim was to investigate the effect of head growth on intelligence growth, while simultaneously assessing the effects of gestational age and birth weight on these growth trajectories. Our results show that the relationship between head growth and IQ development is very similar in both VP/VLBW and term individuals, but gestational age and birth weight are important factors to predict these trajectories. Standardized head circumference scores would control for gestational age but take away the variation in IQ development explained by this important factor; thus, we used raw values to model head growth over time, while timing of assessments at 5 and 20 months was adjusted for gestational age at birth as recommended in clinical practice (Wilson-Ching, Pascoe, Doyle, & Anderson, Reference Wilson-Ching, Pascoe, Doyle and Anderson2014).
We were able to explain >70% of the variation in adult intelligence; however, the majority of this was due to the longitudinal stability of IQ prediction from 6 years of age, not head circumference directly. Nevertheless, a considerable 24% of variance was explained in adult IQ if only the isolated effects of HC at birth and head growth were included in the model (however, this percentage of variance explained was mainly due to the VP/VLBW individuals in the sample). Intelligence is a multidimensional construct that develops through cascades (Bornstein, Hahn, & Wolke, Reference Bornstein, Hahn and Wolke2013). This is reflected in our models, with early development directly and indirectly affecting what comes later, that is, long-term effects of early head growth as marker for brain volume and function on adult IQ.
Full scale IQs are relatively stable over time (Schneider, Niklas, & Schmiedeler, Reference Schneider, Niklas and Schmiedeler2014), but VP/VLBW children often have limited neurocognitive resources (Jaekel, Baumann, & Wolke, Reference Jaekel, Baumann and Wolke2013) and, as a result, their average low IQs remain relatively stable from early childhood onward. Indeed, moderate prediction of adult intelligence is possible from toddler age in VP/VLBW or extremely preterm, but only from 6 years in healthy term comparison children (Breeman et al., Reference Breeman, Jaekel, Baumann, Bartmann and Wolke2015). This is reflected in our multi-group models, as VP/VLBW individuals’ head circumference and growth had their strongest effects on 6 year IQ and from there on indirectly into adulthood, suggesting a rather fixed neurocognitive trajectory (Breeman et al., Reference Breeman, Jaekel, Baumann, Bartmann and Wolke2015; Eryigit Madzwamuse et al., Reference Eryigit Madzwamuse, Baumann, Jaekel, Bartmann and Wolke2015).
Similarly, the effects of family SES at birth on later IQ were larger in the healthy term born group than in VP/VLBW (e.g., .26 vs. .06 at 6 years), indicating differences in developmental plasticity. Family SES affects cognitive development via both genetic and environmental pathways, but VP/VLBW may be more strongly affected by neonatal factors (Jaekel et al., Reference Jaekel, Baumann and Wolke2013) that affect brain and head growth. Intelligence is only one marker of cognitive function, and while VP/VLBW individuals may achieve IQ test results within the average range, their daily performance in school and at work may be affected by other cognitive problems such as attention, executive function, or processing speed.
Finally, IQ assessments changed from childhood to adulthood but our instruments have been shown to deliver reliable and consistent age-appropriate estimations of intelligence (Melchers & Preuss, Reference Melchers and Preuss1991). Moreover, potential variations in IQ estimates across different tests (e.g., Griffiths vs. Bailey Scales) were not of concern due to the prospective inclusion of a matched healthy term comparison group with the same measures taken at all assessment points.
Model fit values indicated that the developmental pathways included in this study accurately reflect the true neurodevelopmental mechanisms in the two populations studied. Thus, our results confirm previous evidence suggesting that repeated early head circumference measurements are a valuable and easy screening tool for long-term neurocognitive risk assessment after preterm birth, in particular in light of recent findings refuting the diagnostic benefits of routine MRI on preterm infants (Edwards et al., Reference Edwards, Redshaw, Kennea, Rivero-Arias, Gonzales-Cinca, Nongena and Counsell2018; Hintz et al., Reference Hintz, Vohr, Bann, Taylor, Das, Gustafson and Higgins2018).
Considering specific neurocognitive mechanisms, however, overall head circumference and brain volume are rather broad markers for cognitive functioning; thus, clinical assessments should always include other tools and a thorough review of known risk factors associated with cognitive impairment. Additional variance in individual IQ development may be explained by regional rather than global differences as well as by alterations in brain connectivity.
ACKNOWLEDGMENTS
Drs. Jaekel, Sorg, Baeuml, Bartmann, and Wolke report no conflicts of interest. This study was supported by the German Federal Ministry of Education and Science (BMBF 01ER0801; PKE24, JUG14). D.W. and P.B. are supported by EU Horizon 2020 (733280; RECAP preterm). The contents are solely the responsibility of the authors and do not necessarily represent the official view of the BMBF. We thank the pediatricians, psychologists, and research nurses who carried out the assessments, and the researchers and administrative staff of the Bavarian Longitudinal Study group who managed the data. We are deeply thankful to our participants for their time and commitment.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S135561771800084X







