INTRODUCTION
An extensive literature that includes population-based, community-based and clinical investigations has documented significantly elevated rates of a variety of neurobehavioral comorbidities in children with epilepsy, including behavioral problems (i.e., depression, anxiety, attention deficit hyperactivity disorder [ADHD]), lower social competence, academic difficulties, and executive dysfunction (Almane et al., Reference Almane, Jones, Jackson, Seidenberg, Koehn, Hsu and Hermann2015; Berg et al., Reference Berg, Vickrey, Testa, Levy, Shinnar and DiMario2007; Hoie et al., Reference Hoie, Mykletun, Sommerfelt, Bjornaes, Skeidsvoll and Waaler2005; Jones et al., Reference Jones, Austin, Caplan, Dunn, Plioplys and Salpekar2008; Lin, Mula, & Hermann, Reference Lin, Mula and Hermann2012; MacAllister & Schaffer, Reference MacAllister and Schaffer2007; Parrish et al., Reference Parrish, Geary, Jones, Seth, Hermann and Seidenberg2007; Rantanen, Eriksson, & Nieminen, Reference Rantanen, Eriksson and Nieminen2012; Russ, Larson, & Halfon, Reference Russ, Larson and Halfon2012). These elevated rates of neurobehavioral comorbidities have been demonstrated in comparison with typically developing children as well as in comparison to children with other medical disorders such as asthma, diabetes, cardiac problems, and other physical problems (Austin et al., Reference Austin, Harezlak, Dunn, Huster, Rose and Ambrosius2001; Austin, Huberty, Huster, & Dunn, Reference Austin, Huberty, Huster and Dunn1998; Davies, Heyman, & Goodman, Reference Davies, Heyman and Goodman2003; Hoare, Reference Hoare1984; McDermott, Mani, & Krishnawami, Reference McDermott, Mani and Krishnawami1995; Rutter, Graham, & Yule, Reference Rutter, Graham and Yule1970).
The timing and course of the neurobehavioral comorbidities in childhood epilepsy have become increasingly clear, and it is now appreciated that many behavioral and cognitive problems can be observed in children with new onset epilepsy, suggesting that these complications are not the consequence of a course of prolonged poorly controlled epilepsy and its treatment or psychosocial complications (Austin et al., Reference Austin, Harezlak, Dunn, Huster, Rose and Ambrosius2001; Fastenau et al., Reference Fastenau, Johnson, Perkins, Byars, deGrauw, Austin and Dunn2009; Oostrom et al., Reference Oostrom, Smeets-Schouten, Kruitwagen, Peters and Jennekens-Schinkel2003). Furthermore, behavioral problems (Austin et al., Reference Austin, Harezlak, Dunn, Huster, Rose and Ambrosius2001), academic struggles (Berg et al., Reference Berg, Smith, Frobish, Levy, Testa, Beckerman and Shinnar2005; McNelis, Johnson, Huberty, & Austin, Reference McNelis, Johnson, Huberty and Austin2005; Overvliet, Aldenkamp, Klinkenberg, Vles, & Hendriksen, Reference Overvliet, Aldenkamp, Klinkenberg, Vles and Hendriksen2011), and rates of specific psychiatric comorbidities (i.e., depression, anxiety, ADHD) (Hesdorffer et al., Reference Hesdorffer, Ludvigsson, Olafsson, Gudmundsson, Kjartansson and Hauser2004; Jones et al., Reference Jones, Watson, Sheth, Caplan, Koehn, Seidenberg and Hermann2007) have been reported even antecedent to the first recognized seizure and epilepsy diagnosis, implicating antecedent neurodevelopmental dysmaturation (Pohlmann-Eden et al., Reference Pohlmann-Eden, Aldenkamp, Baker, Brandt, Cendes, Coras and Hermann2015).
An issue of increasing interest is the degree to which there may be aggregation of neurobehavioral contributions in unaffected siblings and even parents of children with epilepsy (Chowdhury et al., Reference Chowdhury, Elwes, Koutroumanidis, Morris, Nashef and Richardson2014; Clarke et al., Reference Clarke, Strug, Murphy, Bali, Carvalho, Foster and Pal2007; Hesdorffer, Caplan, & Berg, Reference Hesdorffer, Caplan and Berg2012; Iqbal et al., Reference Iqbal, Caswell, Muir, Cadden, Ferguson, Mackenzie and Duncan2015; Levav et al., Reference Levav, Mirsky, Herault, Xiong, Amir and Andermann2002; Smith et al., Reference Smith, Kavros, Clarke, Dorta, Tremont and Pal2012). It is possible that genetics and/or family environment may contribute to the observed increased risk of cognitive and behavioral problems in children with epilepsy. While comparisons of probands with epilepsy to unaffected siblings have been shown to exhibit increased rates of neurobehavioral comorbidities in children with epilepsy, including children with new onset epilepsy (e.g., Austin et al., Reference Austin, Harezlak, Dunn, Huster, Rose and Ambrosius2001; Berg et al., Reference Berg, Vickrey, Testa, Levy, Shinnar and DiMario2007), other studies have included unrelated controls from which unaffected siblings have been found to differ as well as the children with epilepsy (Aronu & Iloeje, Reference Aronu and Iloeje2011; Chowdhury et al., Reference Chowdhury, Elwes, Koutroumanidis, Morris, Nashef and Richardson2014; Iqbal et al., Reference Iqbal, Caswell, Muir, Cadden, Ferguson, Mackenzie and Duncan2015). In the epilepsy literature, studies that demonstrate neuroimaging differences in unaffected siblings that mirror or approach those seen in the probands with epilepsy (Alhusaini et al., Reference Alhusaini, Scanlon, Ronan, Maguire, Meaney, Fagan and Cavalleri2013; Badawy, Vogrin, Lai, & Cook, Reference Badawy, Vogrin, Lai and Cook2013; Wandschneider et al., Reference Wandschneider, Centeno, Vollmar, Symms, Thompson, Duncan and Koepp2014), also raise the question of a contribution of familial relatedness.
Compared to prior investigations (McNelis et al., Reference McNelis, Johnson, Huberty and Austin2005; Sherman, Slick, Connolly, & Eyrl, Reference Sherman, Slick, Connolly and Eyrl2007), we investigate a broader range of potential neurobehavioral comorbidities (Hesdorffer et al., Reference Hesdorffer, Ludvigsson, Olafsson, Gudmundsson, Kjartansson and Hauser2004; McNelis et al., Reference McNelis, Johnson, Huberty and Austin2005; Sherman et al., Reference Sherman, Slick, Connolly and Eyrl2007). In the current study, we examined parent-reported behavior problems and social competence, rates of DSM-IV ADHD, academic problems, and markers of executive dysfunction, all of which have been reported to be elevated in children with epilepsy, including new-onset epilepsy. By comparing children with epilepsy with unaffected siblings and typically developing controls, we sought to understand which comorbidities appear to be influenced by family relatedness and which are independent of family relatedness. Consistent with prior research, we hypothesize that the siblings of children with epilepsy will have lower rates of comorbidities and behavior problems compared to children with epilepsy, but higher rates than the healthy controls.
METHODS
Participants
Research participants consisted of 346 children aged 8–18 years, including youth with recent-onset epilepsy (probands, n=180), their unaffected siblings (n=67), and healthy first-degree cousin controls (n=99). All participants attended regular schools at the time of the study assessment visit. Children with epilepsy were recruited from pediatric neurology clinics at three Midwestern medical centers (University of Wisconsin-Madison, Marshfield Clinic, Dean Clinic) and met the following inclusion criteria: (i) diagnosis of epilepsy within the past 12 months; (ii) no other developmental disabilities (e.g., intellectual impairment, autism); (iii) no other neurological disorder, and (iv) a brain MRI scan obtained as part of routine clinical care that was interpreted as normal. All children entered the study with active epilepsy diagnosed by their treating pediatric neurologists and confirmed by medical record review by the research study pediatric neurologist. We did not exclude children on the basis of psychiatric comorbidities (including ADHD) or learning disabilities. In general, we tried to stay true to the concept of “epilepsy only” as defined broadly in the literature by normal neurological examinations, intelligence, and attendance at regular schools.
Each child’s epilepsy syndrome was defined in a research consensus meeting by the research pediatric neurologists who reviewed all available clinical data (e.g., seizure description and phenomenology, electroencephlogram, clinical imaging, neurodevelopmental history) while blinded to all research cognitive, behavioral, and neuroimaging data. Two levels of epilepsy syndrome classification were undertaken and confirmed by two board-certified pediatric neurologists who were blinded to all research data. Children with epilepsy were first classified into broad syndrome groups including generalized epilepsies (GE) and focal epilepsies (FE), followed by classification into specific GE syndromes [juvenile myoclonic epilepsy (JME), childhood and juvenile absence (Absence), and GE not otherwise specified (NOS)] and FE [benign epilepsy with centrotemporal spikes (BECTS), temporal lobe epilepsy (TLE), frontal lobe epilepsy (FLE), benign occipital epilepsy (BOE), and FE not otherwise specified (NOS)].
First-degree cousins were used as controls, and exclusion criteria were as follows: (i) history of any initial precipitating insult (e.g., simple or complex febrile seizures, cerebral infections, perinatal stroke); (ii) any seizure or seizure-like episode; (iii) diagnosed neurological disease; (iv) loss of consciousness greater than 5 min; (v) other family history of a first-degree relative with epilepsy or febrile convulsions. Unaffected siblings comprised all available siblings of the participants with epilepsy ranging in age 8–18 years meeting criteria (i), (ii), and (iii) specified for the healthy controls. Demographic characteristics of the participants are provided in Table 1.
Table 1 Sample demographics

a Note. Focal epilepsy syndromes: BECTS (n=41), BOE (n=2), TLE (n=19), FLE (n=9), FE Nos (n=19).
b Generalized epilepsy syndromes: Absence (n=27), JME (n=37), GE Nos (n=20).
We have demonstrated that use of first degree cousins as controls does not confer bias. It is possible that, compared to population controls, first degree cousins may share genetic predisposition to cognitive and behavioral problems. If this were the case, one would anticipate that there would be a degree of association between children with epilepsy and controls across measures of cognition, behavior, and even brain structure. In a recent investigation (Hanson et al., Reference Hanson, Morrison, Jones, Jackson, Almane, Seidenberg and Hermann2017) we compared 37 children with new onset epilepsy and all their enrolled cousin controls across 42 measures of cognition, behavior and brain imaging (cortical, subcortical, and cerebellar volumes).
Of the 42 uncorrected correlations involving cognitive, behavioral and neuroimaging measures, the median correlation was 0.06. Looking more specifically at the measures of cognition/behavior and imaging, the median correlations were 0.08 and 0.05, respectively, all approaching 0. Given the lack of association between cases and first degree cousin performances on measures of cognition, behavior, and neuroimaging, the results suggest at most a very weak genetic influence on control group performance, inferring that first-degree cousins serve as unbiased controls for cognitive, behavioral, and neuroimaging research in pediatric epilepsy.
This study was reviewed and approved by the Institutional Review Board of each institution. On the day of study participation, families and children gave informed consent and assent, respectively, and all procedures were consistent with the Declaration of Helsinki (1991) (World Medical Association Declaration of Helsinki, 1991).
Procedures
For this investigation, parents of children with new-onset epilepsy and healthy controls completed structured interviews and questionnaires characterizing the participating child’s gestation, delivery, neurodevelopment, and seizure history. To determine rates of academic services, parents were questioned through a structured interview about their child’s school progress and, in particular, specific educational services provided to address academic problems. These services included the traditional individualized educational plan (IEP) or 504 plan, as well as early childhood interventions including speech therapy, physical therapy, occupational therapy, mandatory summer school, grade retention, special tutoring services (e.g., Title 1 reading), and other specific educational services. This interview was conducted blind to cognitive and behavioral results. Participating child and parent were interviewed separately and completed the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) (Kaufman, Birmaher, Brent, & Rao, Reference Kaufman, Birmaher, Brent and Rao1997). Sibling ADHD diagnosis was determined through the parent K-SADS interview for siblings of children with epilepsy who met the study criteria.
All parents completed the Child Behavior Checklist for children age 6–18 (CBCL/6-18) from the Achenbach System of Empirically Based Assessment (ASEBA) (Achenbach & Rescorla, Reference Achenbach and Rescorla2001) and the Behavior Rating Inventory of Executive Function (BRIEF) (Gioia, Isquith, Guy, & Kenworthy, Reference Gioia, Isquith, Guy and Kenworthy2000). In addition, parents of children with epilepsy provided neurodevelopmental and academic history, and completed CBCL and BRIEF questionnaires for all applicable unaffected siblings. All pertinent medical records for children with epilepsy were obtained after signed release of information was obtained from the parent.
The dependent variables of interest were as follows: (i) Total Competence (higher scores indicating higher level of social competence) and Total Problems (higher scores indicating higher number of behavioral problems) summary scales from parent completed CBCL/6-18; (ii) Metacognition Index (MI) and Behavior Regulation Index (BRI) scales from parent completed BRIEF; (iii) history of education and academic services; and (iv) lifetime ADHD diagnosis as determined by K-SADS. CBCL and BRIEF variables were continuous, while ADHD diagnosis and academic services were dichotomous (yes/no). As a result of the group mean scores for CBCL and BRIEF scales mainly falling in a range that is not considered “clinically significant,” we also conducted secondary analyses examining the proportion of clinically elevated scores for Total Competence, Total Problems, MI, and BRI scales.
The CBCL Total Competence scale consists of items assessing the child’s involvement in activities (i.e., sports, hobbies, and house duties), their social interactions (i.e., involvement in extracurricular activities at school, interactions with friends, and social behaviors), and general academic performance. Higher Total Competence T-scores indicated higher level of function with scores of 35 and lower indicating a clinical level of impairment. The Total Problems scale is made up of two subscales: Internalizing and Externalizing Problems. Internalizing Problems consist of items assessing anxiousness, being withdrawn or depressed, and somatic complaints (i.e., nightmares, fatigue, stomach complains, and headaches). Externalizing Problems include items assessing rule breaking (i.e., swearing, stealing, and truancy from school) and aggressive behaviors (i.e., arguing, bullying, and threatening others). Higher Total Problems T-scores indicate higher level of impairment with scores of 65 and higher indicating clinical significance. Despite the disparate items, the T-scores for Internalizing and Externalizing sub-scales are highly correlated (current sample: R2=0.67; p<.01), and as a result we have chosen to examine Total Problems rather than the individual sub-scales.
The BRIEF BRI measures the child’s ability to shift their cognitive focus and modulate their emotions/behaviors through appropriate inhibitory controls. The MI assesses the child’s ability to initiate, plan, organize, and preserve future-oriented problem solving in working memory. Higher T-scores on BRI and MI indicate lower level of function and scores of 65 and higher are considered of potential clinical significance.
Statistical Analyses
To accommodate our study design with multiple children per family, linear mixed effect models with random intercepts (McCulloch, Searle, & Neuhaus, Reference McCulloch, Searle and Neuhaus2008) were used for analysis of the four continuous variables using SAS 9.4 PROC MIXED, and logistic regression models with normally distributed random intercepts (McCulloch et al., Reference McCulloch, Searle and Neuhaus2008) were used for the two binary outcomes using STATA 15 xtlogit. Each model treated group (Epilepsy, Siblings, Controls) as a factor to test for among group differences in a global 2 df test. Age and gender were adjusted as covariates. The analyses described above involved six tests; to control family-wise, or overall, Type I error rate, at alpha=0.05, we used the Bonferroni procedure to adjust for multiple comparisons, declaring group differences as significant when p<.05/6. Post hoc pair-wise comparison tests were only performed when overall tests remained significant after the Bonferroni procedure. Model results are presented in terms of group-specific proportions or means, using fitted models to adjust to the age-by-sex distribution of the overall sample, and averaging out random effects.
To examine whether there were significant group differences on rates of scores classified as clinically significant, CBCL Total Competence and Total Problems, and BRIEF Behavioral Regulation Index and Metacognition Index, T-scores were recoded as dichotomous variables (clinically significant: yes or no). Pearson’s Chi Square was used to assess among group differences on the clinical significance of the T-scores.
RESULTS
There were significant differences (all p≤.003 before Bonferroni adjustment) between the three groups on all six dependent variable endpoints (Table 2); as such, pairwise comparisons were conducted for each dependent variable.
Table 2 Marginal proportions (P), marginal means (T-score), and standard errors (SE) by group

Note. a,b,c Denotes significant difference among groups (p<.05): a=controls, b=siblings, c=children with epilepsy.
* Estimates are adjusted to the age x sex distribution across the entire sample after linear or logistic random effects model fitting.
** Chi-square or F statistic, along with p-value, for testing any difference among the three groups, before Bonferroni correction; test results from logistic (P) or linear (M) random effects model fits.
Lifetime ADHD Diagnosis and Academic Service Rates
Age- and sex-adjusted lifetime ADHD diagnosis rates were significantly higher for children with epilepsy (29%) compared with their unaffected siblings (13%; p<.02) and controls (8%; p<.001). The group difference in rates of ADHD between unaffected siblings and controls (p=.38) was not significant, although estimates were somewhat different. Adjusted proportions and standard errors are listed in Table 2.
Academic service rates were significantly higher for children with epilepsy (51%) compared with their healthy siblings (31%; p=.01) and controls (18%; p<.001). The difference in rate of academic service use between healthy siblings and controls (p=.07) was suggestive of a difference, but not significant. Adjusted proportions and standard errors are listed in Table 2. A summary of the rates of specific type of academic services received by group are listed in Table 3.
Table 3 Lifetime academic services rates by group

CBCL Total Competence and Total Problems
Age- and sex-adjusted Total Competence was significantly higher for controls (M=52.3) compared with both children with epilepsy (M=44.6; p<.001) and their unaffected siblings (M=45.9; p<.001). The difference between children with epilepsy and their unaffected siblings was small and not significant (p=.22). While differences among groups were detected on mean scale scores, the means were generally in the average range. As such, rates of clinically significant T-scores for Total Competence were also examined and found to be significantly different between the groups (χ2(2, N=342)=13.45; p<.001). Only 14% of healthy controls had clinically significant T-scores compared to 28% of siblings and 35% of probands with epilepsy.
Age- and sex-adjusted Total problems were significantly lower for controls (M=45.8) compared with both children with epilepsy (M=56.2; p<.001) and their unaffected siblings (M=50.5; p<0.05). Children with epilepsy also had significantly higher Total Problems scores than their unaffected siblings (p<0.001). Mean scores for Total Competence and Total Problems are summarized in Figure 1. Again, because these means were generally in the average range, rates of clinically significant T-scores for Total Problems were also examined and found to be significantly different among the groups (χ2(2, N=343)=22.31; p<.001). Only 7% of healthy controls and 3% of unaffected siblings had clinically significant T-scores compared to 24% of probands with epilepsy.
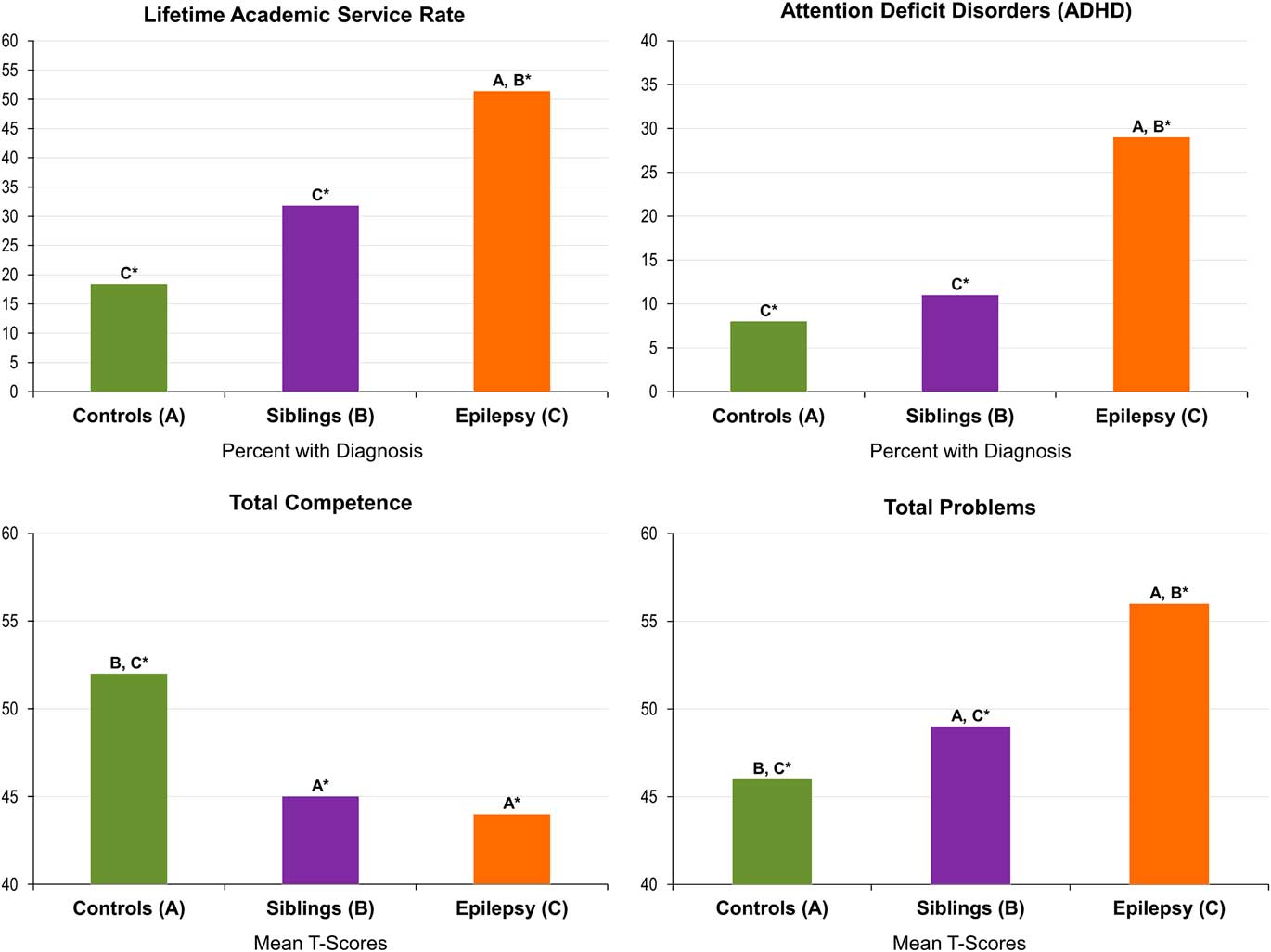
Fig. 1 Rates of comorbidity in children with epilepsy, their siblings, and controls. *A, B, C denotes significant differences among groups: A=controls, B=siblings, C=epilepsy.
BRIEF Behavior Regulation Index and Metacognition Index
The BRI score was significantly higher for children with epilepsy (age- and sex-adjusted M=53.5) compared with their unaffected siblings (M=48.0; p<.001) and controls (M=46.5; p<.001). No significant differences were found between unaffected siblings and controls (p=.35). Rates of clinically significant T-scores for the Behavior Regulation Index were significantly different among the groups (χ2(2, N=346)=17.94; p<.001). Only 5.1% of healthy controls and 4.5% of unaffected siblings had clinically significant T-scores compared to 20% of probands with epilepsy.
The Metacognition Index was significantly higher for children with epilepsy (age-and-sex adjusted M=56.4) compared with their unaffected siblings (M=50.3; p<.001) and controls (M=48.0; p<.001). No significant differences were found between their unaffected siblings and controls (p=.19). Rates of clinically significant T-scores for the Metacognition Index were significantly different among the groups (χ2(2, N=346)=27.60; p<.001). Only 5.1% of healthy controls and 7.5% of unaffected siblings had clinically significant T-scores compared to 27.2% of probands with epilepsy.
Overall, there appeared to be an effect of epilepsy, unassociated with family relatedness, in regard to rates of ADHD as well as executive dysfunction including behavioral regulation and metacognition, reflected by significantly higher rates of abnormality in the children with epilepsy compared with both unaffected siblings and controls, with much weaker differences between the latter two groups (Figure 1).
DISCUSSION
To determine the relative contributions of epilepsy and familial aggregation to neurobehavioral comorbidities commonly associated with childhood epilepsies, we compared children with epilepsy, their unaffected siblings, and typically developing controls across a broad range of comorbidities including ADHD, academic problems, parent-reported social competence and behavioral problems as well as assessments of day-to-day executive function. To our knowledge, this is the broadest examination to date of the relationship between family relatedness and diverse behavioral and academic complications commonly reported among children with epilepsy.
We hypothesized that the siblings of children with epilepsy will have lower rates of comorbidities and behavior problems compared to children with epilepsy, but higher rates than the healthy controls. Of interest, varying patterns of association with the comorbidity measures were demonstrated. Specifically, there appeared to be an effect of epilepsy, unassociated with family relatedness, in regard to rates of ADHD as well as executive dysfunction including behavioral regulation and metacognition, reflected by significantly higher rates of abnormality in the children with epilepsy compared with both unaffected siblings and controls, with much weaker differences between the latter two groups (Figure 1). These differences were reflected both in terms of mean score differences as well as rates exceeding critical cut points. Our findings of elevated rates of ADHD and executive dysfunction in children with epilepsy compared to controls are consistent with previous literature (Berl et al., Reference Berl, Terwilliger, Scheller, Sepeta, Walkowiak and Gaillard2015; MacAllister et al., Reference MacAllister, Vasserman, Vekaria, Miles-Mason, Hochsztein and Bender2012; Sherman et al., Reference Sherman, Slick, Connolly and Eyrl2007), the current results obtained in children with new onset epilepsies and also suggesting the contribution of family relatedness.
There appeared to be a broader contribution of family relatedness to measures of social competence and behavioral problems. Consistent with the literature that has compared children with epilepsy to either siblings or healthy controls, lower competence and higher total behavioral problems were evident in the children with epilepsy compared with both unaffected siblings and typically developing controls (Aronu & Iloeje, Reference Aronu and Iloeje2011; Austin et al., Reference Austin, Harezlak, Dunn, Huster, Rose and Ambrosius2001). However, unaffected siblings exhibited significantly lower competence and higher behavioral problems compared to the controls, consistent with more recent findings suggesting a contribution of family relatedness, reflecting an impact of genetic, social, or other factors (Hesdorffer et al., Reference Hesdorffer, Caplan and Berg2012).
Specifically, the presence of an effect of family relatedness or family aggregation leads to the question of the etiology of the effect. Quite often the presence of an effect of family aggregation leads to speculation regarding possible genetic influences even though no genetic material has been collected or analyzed. A competing hypothesis is that family aggregation may be related to “environmental” factors such as family cohesion, parenting styles, social disadvantage, or other factors. An important direction for the future is to parse out the contribution of these competing etiologies for those dependent measures shown to reliably result in “familial aggregation”.
Finally, regarding academic problems, children with epilepsy again had higher rates of supportive academic services than unaffected siblings and controls, and a trend was observed of more problems in the unaffected siblings compared with controls (51% vs. 31% vs. 18%). While previous studies support findings of elevated academic service rates in children with epilepsy (Almane et al., Reference Almane, Jones, Jackson, Seidenberg, Koehn, Hsu and Hermann2015; Berg et al., Reference Berg, Smith, Frobish, Levy, Testa, Beckerman and Shinnar2005; Overvliet et al., Reference Overvliet, Aldenkamp, Klinkenberg, Vles and Hendriksen2011; Sogawa, Masur, O’Dell, Moshe, & Shinnar, Reference Sogawa, Masur, O’Dell, Moshe and Shinnar2010), the noted elevated rates of academic services in epilepsy siblings is a unique finding of the current study. Academic service rates by group are summarized in Figure 1. Sample rates by the specific type of academic services received are listed in Table 3.
In the broader child epilepsy literature, a question has been raised regarding the degree to which proxy-based reports (e.g., from parents) accurately reflect the emotional–behavioral status of their children with epilepsy (Eom, Caplan, & Berg, Reference Eom, Caplan and Berg2016), specifically suggesting that parent-proxy behavior measures such as the CBCL are contaminated by the emotional impact of epilepsy on the parents themselves. In contrast to this perspective, we have found parent reports of competence, behavioral problems, academic difficulties and ADHD as assessed here to have direct neuroanatomic correlates in the children (Dabbs, Jones, Jackson, Seidenberg, & Hermann, Reference Dabbs, Jones, Jackson, Seidenberg and Hermann2013; Hermann et al., Reference Hermann, Jones, Sheth, Dow, Koehn and Seidenberg2006; Saute et al., Reference Saute, Dabbs, Jones, Jackson, Seidenberg and Hermann2014).
Specifically, parent reports of higher (better) social competence skills are associated with increased cortical thickness, especially in frontal regions. Parent reports of behavioral problems are associated with patterns of decreased cortical thickness that vary as a function of the specific behavioral issue under investigation. Thus, the parent-report version of the CBCL is associated with variations in cortical thickness among children with epilepsy with anatomic abnormalities specific to selected competence and behavioral problem scales, with more reliable and robust patterns of thinning across scales assessing externalizing behaviors, with generally less prominent findings on scales assessing internalizing behaviors. Parents’ observations and reports of competence and behavior problems in their children have direct neurobiological correlates in the brains of their children and hence are an important area of inquiry.
The ecological implications of these findings reported here suggest that in clinical work there is significant utility to obtaining a broad familial history which will serve as an important component of understanding the neurobehavioral comorbidities of a child with epilepsy. We focused here on the status of siblings, but broader familial investigation (e.g., parents) is likely pertinent not only to behavioral issues and quality of life (Mendes, Crespo, & Austin, Reference Mendes, Crespo and Austin2017), but to cognition and academic performance as well (Fastenau et al., Reference Fastenau, Shen, Dunn, Perkins, Hermann and Austin2004) as is increasingly appreciated.
LIMITATIONS AND FUTURE DIRECTIONS
Several limitations and opportunities for future research are associated with this investigation. First, due to limited sample size we were not able to examine whether comorbidity rates or behavior problems differed for siblings of children with focal vs. generalized epilepsy. One might hypothesize that children with the “genetic” generalized epilepsies may be more likely to have siblings with targeted comorbidities than children with focal epilepsies, but this remains to be determined. Second, again due to limited sample size, we were not able to examine group differences for the specific type of academic services (IEP, Birth-Age 3, Early Childhood, and School Services). Third, many of the contrasts of group means, while revealing significant differences among groups, remained in the generally average or normal range. Thus, examination of critical clinically meaningful cut points is essential to gauge the clinical significance of the findings, as was done here as well. Fourth, the presence of an effect of family relatedness or family aggregation leads to the question of the etiology of that effect, something which we cannot untangle here but which is important for future research. Quite often the presence of an effect of family aggregation leads to speculation regarding possible genetic influences even though no genetic material has been collected or analyzed.
A competing hypothesis is that family aggregation may be related to “environmental” factors such as family cohesion, parenting styles, social disadvantage, or any number of other factors. An important direction for the future is to parse out the contribution of these competing etiologies for those dependent measures shown to reliably result in “familial aggregation”. Fifth, our sample consists of children with uncomplicated idiopathic epilepsies. How these findings would translate to a cohort of children with more complicated and treatment resistant epilepsies remains to be determined. Sixth, the siblings did not undergo formal neuropsychological assessment and direct comparison of cognitive status would be very valuable going forward. Finally, the sample size in the control and sibling groups was more limited, so while we were able to reliably detect differences between the epilepsy group and either of the other two groups, there were suggestions of differences between control and sibling groups that did not reach statistical significance.
In conclusion, examining a broad range of potential comorbidities of childhood epilepsy, there appears to be variable influence of epilepsy compared to familial relatedness across the problems of interest. There appears to be no simple and generalizable relationship between the neurobehavioral comorbidities of childhood epilepsy and the risk conferred by the presence of epilepsy in comparison to family relatedness. Some comorbidities are clearly associated with the presence of epilepsy independent of family relatedness, other comorbidities show a contribution of family relatedness, and there was no area of comorbidity where there were no differences among groups.
The mechanism(s) underlying elevated comorbidities in unaffected siblings remains to be determined and may reflect a genetic contribution, the effects of family environment, or other factors. Untangling the path by which family relatedness influences comorbidity risk is an important task for the future (Badawy et al., Reference Badawy, Vogrin, Lai and Cook2013; Clarke et al., Reference Clarke, Strug, Murphy, Bali, Carvalho, Foster and Pal2007; Levav et al., Reference Levav, Mirsky, Herault, Xiong, Amir and Andermann2002; Smith et al., Reference Smith, Kavros, Clarke, Dorta, Tremont and Pal2012). The degree of risk associated with epilepsy relative to family relatedness appears to vary across specific individual comorbidities.
ACKNOWLEDGMENTS
Disclosure of Conflicts of Interest: None of the authors have any conflict of interest to disclose. Financial Support: This work was supported by NIH 3RO1-44351 and the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS), grant 9U54TR000021. The funding sources had no role in study design; in collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. We thank Raj Sheth, MD, and Lucyna Zawadzki, MD, for their contributions to the study. Also greatly appreciated are Melissa Hanson and Kate Young for overall study coordination, participant recruitment, cognitive assessment, and data management.






