Published online by Cambridge University Press: 12 June 2012
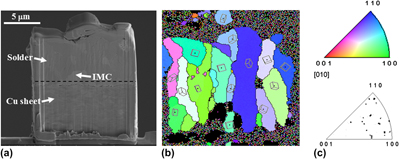
To enhance the reliability of Pb-free solders in high temperature and high humidity conditions, the minor alloying elements of Be and Co are investigated in terms of the growth of Sn whiskers and various properties of Sn-based Pb-free solders. Sn whisker growth is suppressed by adding up to 0.02 wt% Be to Sn-based solders. Adding Be and Co can effectively reduce the undercooling of Sn–1.0Ag–0.5Cu (wt%) solders. And the microstructures of Sn–1.0Ag–0.5Cu–0.02Be solders are similar to those of Sn–1.0Ag–0.5Cu. Furthermore, adding Co to solders increases the microhardness number as a result of the solid solution hardening. Adding Be causes no changes in the morphology or thickness of Cu6Sn5 at the Cu/OSP (organic solderability preservative) under bump metallurgy interface. However, the scallop-like Cu6Sn5 microstructure changes to a flat (Cu,Co)6Sn5 microstructure when 0.05 wt% of Co is added to Sn–1.0Ag–0.5Cu–0.02Be solders.