Published online by Cambridge University Press: 20 May 2020
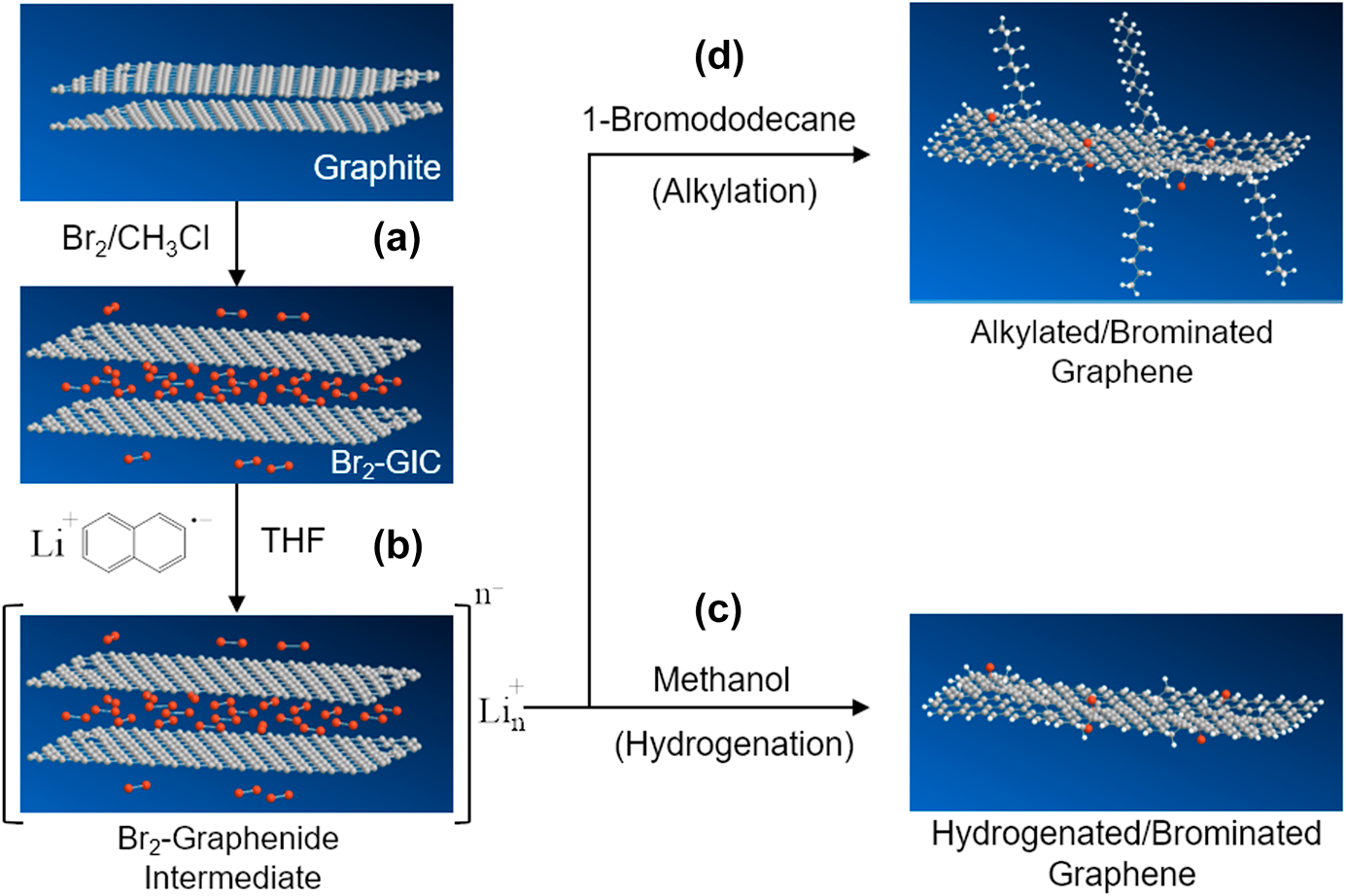
Developing easy and effective surface functionalization approaches has required to facilitate the processability of graphene while seeking novel application areas. Herein, an in situ single-step reductive covalent bromination of graphene has been reported for the first time. Highly brominated graphene flakes (>3% Br) were prepared by only subjecting the bromine-intercalated graphite flakes to a reduction reaction with reactive lithium naphthalide. The bromine-functionalized graphene was characterized by X-ray photoelectron spectroscopy and thermogravimetric analysis. Results revealed that Br2 molecules acted as both an intercalating agent for the graphite and a reactant for the surface functionalization of the graphene. After brominating, the remaining negative charges on the reduced graphene surface were further used for the dual surface functionalization of graphene with a long-chain alkyl group (~1% dodecyl group addition). The functionalized graphenes were also characterized by Fourier transform infrared and Raman spectroscopy.