Introduction
Donkeys (Equus asinus) are traditionally used as working animals for transportation and farm activities in developing countries (Ali et al., Reference Ali, Baber, Hussain, Awan and Nadeem2014). In Europe, donkey populations have declined from the 1960s and nowadays, according to the Food and Agriculture Organization, the population is estimated to be about 395,910 animals (FAO, 2020). In Italy, the donkey population has gradually decreased due to industrialization and urbanization from 500,000 in the 1960s to about 23,000 in the early 2000s (FAO, 2020).
In recent years, donkeys have found increasing use in leisure activities, onotherapy (animal-assisted therapy) and milk production (Veneziano et al., Reference Veneziano, Di Loria, Masucci, Di Palo, Brianti and Gokbulut2011). This has caused an increase of the Italian donkey population to currently 92,965 donkeys (AIA, 2020). In Italy, there is a long-established tradition in donkey breeding as confirmed by the presence of several autochthonous breeds such as Amiatino, Asinara, Martina Franca, Ragusano, Romagnolo, Pantesco, Sardo, Grigio Siciliano and Viterbese (Bigi & Zanon, Reference Bigi and Zanon2008). However, despite the economic and sanitary relevance of parasitosis in farm animals, there are studies on ectoparasites and protozoa, but limited epidemiological data regarding helminths affecting this species available in the scientific literature (Matthews & Burden, Reference Matthews and Burden2013).
In general, all common helminth parasites of horses also infect donkeys (Matthews & Burden, Reference Matthews and Burden2013), and equine intestinal strongyles are considered ubiquitous in grazing equids worldwide, so co-grazing animals can act as a source of infection for both species (Matthews & Burden, Reference Matthews and Burden2013). Similar to horses, the most important parasitic nematodes of donkeys are Strongylidae (large strongyles – Strongylus vulgaris, Strongylus edentatus, Strongylus equinus, Strongylus asini – and small strongyles – Cyathostominae). Other relevant helminths are ascarids (Parascaris spp.), pinworms (Oxyuris equi), threadworms (Strongyloides westeri), tapeworms (Anoplocephala spp.) and lungworms (Dictyocaulus arnfieldi) (Matthews & Burden, Reference Matthews and Burden2013).
Donkeys represent the main competent host and reservoir for D. arnfieldi and the major source of pasture contamination for horses (Beelitz et al., Reference Beelitz, Gobel and Gothe1996), although infection from horse to horse is reported (Matthews, Reference Matthews and Burden2002). Clinical signs in donkeys are quite rare, but in horses, tachypnea and persistent cough could be present (Matthews, Reference Matthews and Burden2002).
Moreover, horses and donkeys can be parasitized by the liver fluke, Fasciola hepatica, in wetter areas (Matthews & Burden, Reference Matthews and Burden2013). Although massive parasitic infections are often subclinical in donkeys, the impact of endoparasites on their health, welfare and production is still unclear (Buono et al., Reference Buono, Roncoroni, Pacifico, Piantedosi, Neola, Barile, Fagiolo, Várady and Veneziano2018). Generally, donkeys with a high parasite load of small strongyles appear healthy, and mainly overworked animals or those with poor physical status show clinical signs, as reported in developing countries where substandard environmental conditions exist (Burden et al., Reference Burden, Du Toit, Hernandez-Gil, Prado-Ortiz and Trawford2010; Matthews & Burden, Reference Matthews and Burden2013; Tavassoli et al., Reference Schneider, Pfister, Becher and Scheuerle2015). Regarding large strongyles, in an eight-year-old donkey, S. vulgaris caused obstruction of the cranial mesenteric artery, which led to death of the animal (Borji et al., Reference Borji, Moosavi and Ahmadi2014).
Anthelmintic therapy represents a main strategy to control endoparasitosis in horses (Nielsen et al., Reference Nielsen, Scare, Gravatte, Bellaw, Prado and Reinemeyer2018), as well as in donkeys (Buono et al., Reference Buono, Roncoroni, Pacifico, Piantedosi, Neola, Barile, Fagiolo, Várady and Veneziano2018); however, only few anthelmintic drugs are registered for use in donkeys, so extra-label use of products registered for horses and ruminants is the norm (Gokbulut & McKellar, Reference Gokbulut and McKellar2018).
Previously in Italy, only three reports on a small number of donkeys (approximately 100 animals) were available about helminth infections (Giannetto et al., Reference Giannetto, Poglayen, Brianti, Giannetto, Poglayen and Brianti2008; Trentini et al., Reference Trentini, Stancampiano, Usai, Micagni and Poglayen2010; Garippa et al., Reference Garippa, Pintore and Sanna Passino2016). In Europe, robust parasitological studies have been carried out by The Donkey Sanctuary (UK) but did not aim to conduct an epidemiological study on a national level (Matthews & Burden, Reference Matthews and Burden2013).
The aims of the present survey were to: (1) determine the prevalence and distribution of main helminth infections in Italian donkeys; (2) investigate the risk factors associated with infection; and (3) describe current parasite control practices adopted for donkeys.
Materials and methods
Study animals
The study was performed during 2014–2016 and included 1775 donkeys raised on 77 farms located in 13 Italian regions (nine farms from northern Italy; 28 from central Italy; 26 from southern Italy; 14 from islands). The sample size was calculated using the formula proposed by Thrusfield (Reference Thrusfield2007), including the following information: study population at the time of the start of the study (58,647 donkeys; data supplied by Italian Breeders Association – AIA, 2013); expected prevalence (80%) based on the results of a regional study on intestinal strongyles in donkeys conducted in Sicily, southern Italy (Giannetto et al., Reference Giannetto, Poglayen, Brianti, Giannetto, Poglayen and Brianti2008); desired absolute precision (3%); and confidence interval (CI) (99%).
The age of donkeys was determined matching owners’ information with examination of teeth, according to the guide proposed by The Donkey Sanctuary (2016). The animals were classified into four age groups: ≤1 year; 1–4 years; 4–10 years; and >10 years. Furthermore, body condition scores (BCS) were determined using the chart developed by The Donkey Sanctuary (Reference Tavassoli, Yamchi and Hajipour2008). Briefly, a scale of 1–5 was applied (1 = poor, 2 = moderate, 3 = ideal, 4 = fat and 5 = obese).
All procedures on the donkeys were performed with the owner's consent and according to the European Communities Council Directive (86/609/EEC). The investigation was approved by the Ethical Animal Care and Use Committee of the University of Naples ‘Federico II’ number 889/04.
Questionnaires and data collection
On each farm, owners were interviewed using a purpose-designed questionnaire to obtain a complete history of the farms and examined animals. The questionnaire consisted of 36 closed-ended questions (multiple choice and yes/no options) and it was divided into three sections (general information, grazing practices and farm management, helminth control procedures). The questionnaire can be found in the supplementary material.
The first section included general information about the farms and donkeys: living area (northern, central, southern Italy and island) as previously defined (Otranto & Dantas-Torres, Reference Nielsen, Branan, Wiedenheft, Digianantonio, Garber, Kopral, Philippi-Taylor and Traub-Dargatz2010), herd size (number of donkeys; small: ≤25; medium: 25–50; large: >50), breed (pure, such as Amiatino, Martina Franca, Ragusano, Romagnolo, or mixed; hybrids like mules and hinnies were not included in this study), sex (male, female, gelding), age (≤1 year; 1–4 years; 4–10 years; >10 years), BCS, primary use (milk production, meat production, onotherapy, recreational activities, milk production for cosmetic industry and pet animal).
The second section included information about farm management: availability of box stalls and access to grazing (yes or no); months of grazing per year; and co-grazing with horses (yes or no) or ruminants (yes or no).
The third section included information about anthelmintic treatments and deworming strategy: the number of treatments per donkey per year (0; 1–2; >2) for each anthelmintic drug type considered (ivermectin (IVM), moxidectin (MOX), benzimidazoles (BZ), pyrantel (PYR), IVM/praziquantel (PZQ), MOX/PZQ, other products).
Coprological analysis
Individual fecal samples were collected from all donkeys included in the study (1775) and, according to general recommendations proposed by Nielsen et al. (Reference Mayhew, Brewer, Reinhard and Greiner2010a), faeces were taken directly from the rectum of each animal, or alternatively picked up off the ground from fresh deposits (especially from nervous animals and foals) using plastic gloves. Fecal samples were stored in portable refrigerators (about 4°C) and individual fecal egg counts (FECs) were performed within 48 h, using a special modification of the McMaster method with a detection limit of ten eggs per gram (EPG) (Zajac & Conboy, Reference Wilkes, Heller, Raidal, Woodgate and Hughes2011). The floatation medium used was the Sheather's sugar solution with a specific gravity of 1.250 (Lester & Matthews, Reference Lester and Matthews2014). Based on visual morphological identification (Zajac & Conboy, Reference Wilkes, Heller, Raidal, Woodgate and Hughes2011), each egg was classified as belonging to intestinal strongyles, Parascaris spp., S. westeri, O. equi, Anoplocephala spp. Furthermore, a qualitative centrifugation/flotation technique (Proudman & Edwards, Reference Pihl, Nielsen, Olsen, Leifsson and Jacobsen1992) using Sheather's sugar solution and a qualitative sedimentation technique (Ambrosi, Reference Ambrosi1991) were used for the detection of Anoplocephala spp. and F. hepatica eggs, respectively.
Fecal cultures and speciation of intestinal strongyle
Pooled fecal cultures (from a maximum of five donkeys) were set up for each farm. Briefly, 20 g of faeces from samples with FECs greater than the suggested 300 EPG selective therapy cut-off (Matthews & Burden, Reference Matthews and Burden2013) were used and then incubated at 27–30°C for 7–10 days for larval development. At the end of the incubation period, third-stage larvae (L3s) were recovered by the Baermann technique (MAFF, 1986) and identified according to their specific genera and/or species, based on the shape and number of gut cells, using the keys proposed by the Atlas of Diagnosis of Equine Strongylidosis (Cernea et al., Reference Cernea, Madeira de Carvalho, Cozma, Cernea, Madeira de Carvalho and Cozma2008). Where possible, up to 100 larvae (L3s) were identified.
Baermann technique and diagnosis of D. arnfieldi
For each donkey, individual Baermann technique was also used to ascertain the occurrence of lung worm infection on 30 g of faeces, in a double layer of gauze, closed with an elastic band and placed in a funnel (Zajac & Conboy, Reference Wilkes, Heller, Raidal, Woodgate and Hughes2011). The funnel was filled with tap water until the faeces were immersed. After 24 h, the content of each Baermann was collected in 15 ml tubes and centrifuged at 400 g for 5 min. The supernatant was discarded, and 1 ml of each pellet was transferred to nematode slides and examined under microscope.
Statistical analysis
Animal-level prevalence was computed with the associated 95% CIs for all the considered parasites (intestinal strongyles, Parascaris spp. and D. arnfieldi) with the exception of S. vulgaris, whose prevalence was estimated at farm level. All the answers from the questionnaires were included as independent variables in a multivariate logistic regression analysis developed by a stepwise regression process to explore factors that could be considered predictors for positive status (>0 EPG) for intestinal strongyles, Parascaris spp. and D. arnfieldi (>0 larvae per gram) at the animal level (sex, breed, presence of pasture, co-pasture with ruminants, co-grazing with horses, BCS, age, living area, herd size and number of treatments/year) and S. vulgaris at the farm level (presence of pasture, co-pasture with ruminants, co-pasture with horses, presence of young stock (donkeys <1 year) living area, herd size and number of treatments/year). Results were presented as adjusted odds ratios. All the statistical analyses were performed using commercial software (SPSS, Version 22.0, Chicago, IL, USA).
Results
Study animals and questionnaire survey
Of 1775 donkeys, 396 (22.3%) were intact males, 1354 (76.3%) were females and 25 (1.4%) were geldings. Most of the donkeys (937, 52.8%) were mixed-breed, while 838 (47.2%) were pure-breed. The majority of donkeys (1133–63.8%) showed an ideal BCS (BCS = 3), 198 (11.2%) had a BCS < 3 and 206 (11.6%) > 3. For 238 (13.4%), BCS was not available. The mean age of animals ± standard deviation was 7.7 ± 5.8 years (range: three months–33 years).
Regarding location of farms, 28/77 (36.4%) were located in central Italy for a total of 693 donkeys (39.0%), 26/77 (33.8%) in southern Italy for a total of 379 donkeys (21.4%), 14/77 (18.2%) in Sicily for a total of 266 donkeys (15.0%) and 9/77 (11.7%) in northern Italy for a total of 437 donkeys (24.6%).
In most of the farms (66/77, 85.7%), donkeys lived at pasture, while in 11/77 (14.3%) donkeys had no access to pasture; 1612 donkeys (90.8%) had access to pasture, while 163 (9.2%) lived in box stalls with access to a small, annexed paddock. Of 1612 donkeys belonging to the 66 farms that had access to pasture, grazing was permanent for 1265 donkeys (78.5%), while seasonal pasture access was provided to 347 donkeys (21.5%). Grazing time ranged from three to 12 months. Mixed/alternate grazing with cattle was reported for 336/1612 donkeys (20.8%), while 797/1612 donkeys (49.4%) co-grazed with horses.
Owners reported that donkeys were involved mainly in milk production for human nutrition (816, 45.9%) and as pet animals (723, 40.7%); other uses included onotherapy (332, 18.7%), recreational outdoor activities (274, 15.4%), meat (153, 8.6%) and milk production for the cosmetic industry (97, 5.4%). Several donkeys were employed in more than one activity and not all owners raised donkeys for reproductive purposes.
Sixty out of 77 (78.0%) farm owners declared to perform anthelmintic treatments, and the overall number of treated donkeys was 1557 (87.7%), while 17 owners (22.0%) did not treat their animals with anthelmintics (218–12.3%). Regarding the treatment scheme, the highest percentage of owners (53.3%) reported deworming their donkeys once a year, followed by 43.3% and 3.3% treating twice and four times/year, respectively; none of the owners treated three times/year. The mean number of anthelmintic treatment/year was 1.4 (range 1–4). Owners declared using different anthelmintic drugs during the year following arbitrary and empirical rotational criteria. Macrocyclic lactones (MLs) (IVM and MOX) were the drugs most frequently administered (84.8%) (IVM 71.8% and MOX 13.0%); sometimes, IVM was administered in association with PZQ (5.5%), whereas no donkey was treated with MOX + PZQ. Other drugs used were BZ (22.4%), PYR (21.3%), PZQ (0.2%) and other active principles such as phytotherapeutic dewormers (3.1%) (fig. 1).
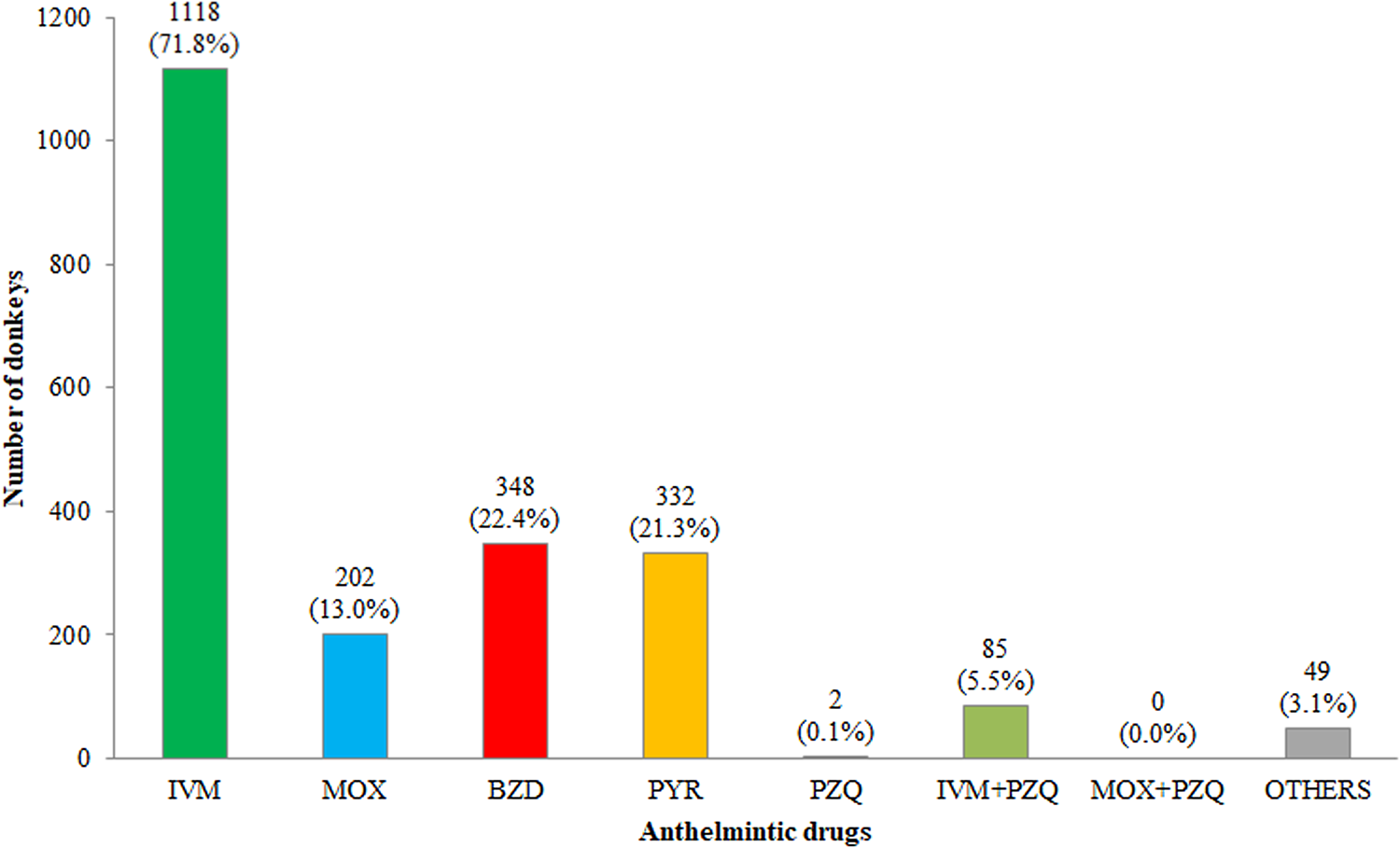
Fig. 1. Anthelmintics administered by donkey owners/breeders. Respondents could use more than one drug. Abbreviations: IVM, ivermectin; MOX, moxidectin; BZD, benzimidazoles; PYR, pyrantel pamoate; PZQ, praziquantel.
Parasitological examinations
In total, 1536/1775 donkeys (86.5%) (95% CI 84.9–88.1) were helminth positive in single or mixed infections; 1271 (82.7%), 251 (16.3%), 10 (0.7%) and 4 (0.3%) were parasitized by one, two, three and four helminth categories, respectively (table 1).
Table 1. Single and mixed helminth infections in study donkeys (N = 1775).
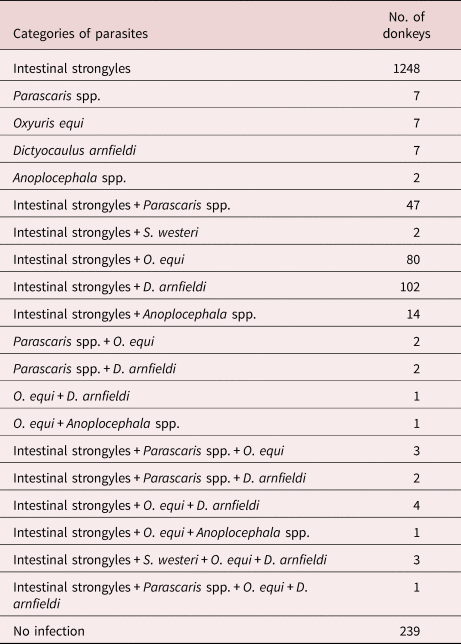
Intestinal strongyle eggs were found in all tested farms (77/77–100%) with a prevalence at animal level of 84.9% (1507/1775) (95% CI 83.2–86.6), with a single strongyle infection in 1248. In 502 positive donkeys (28.3%), strongyle FEC was lower than the recommended donkey cut-off for selective anthelmintic therapy (300 EPG). The average EPG was 706.4 ± 747.8 (min 10–max 9200). Regarding the egg shedding level, 43.4% of donkeys had a FEC < 300 EPG (low contaminators), 19.1% had a FEC ranging between 300 and 600 EPG (moderate contaminators) and 37.5% had a FEC > 600 EPG (high contaminators) (fig. 2). The infection rate for intact males, females and geldings was 85.1%, 85.1% and 72.0%, respectively. No significant differences related to sex were found. The infection rate of Parascaris spp. was 3.6% (64/1775 donkeys) (95% CI 2.8–4.5) and the average EPG was 391.6 ± 525.7 (min 10–max 2600) with a farm prevalence of 30.0%.

Fig. 2. Number and percentage of donkeys belonging to strongyle egg shedding categories. EPG, eggs per gram.
Faecal cultures revealed the presence of larvae (L3s) of Cyathostomum sensu lato (100%) followed by S. vulgaris (31.0%), Poteriostomum spp. (25.0%), Triodontophorus spp. (9.0%), S. edentatus (7.0%) and S. equinus (5.0%).
Dictyocaulus arnfieldi larvae were reported in 122 donkeys (6.9%; CI 5.7–8.1) with a farm prevalence of 24.7%. The mean number of larvae was 57.5 (min 1–max 285).
Oxyuris equi, Anoplocephala spp. and S. westeri eggs were reported in 103 (5.8%; CI 4.7–6.9%), 18 (1.0%; CI 0.6–1.5%) and in five (0.3%; CI 0.1–0.5%) donkeys, with a farm prevalence of 24.7%, 6.5% and 3.9%, respectively. None of the examined donkeys were positive for liver fluke F. hepatica.
Risk factor analyses
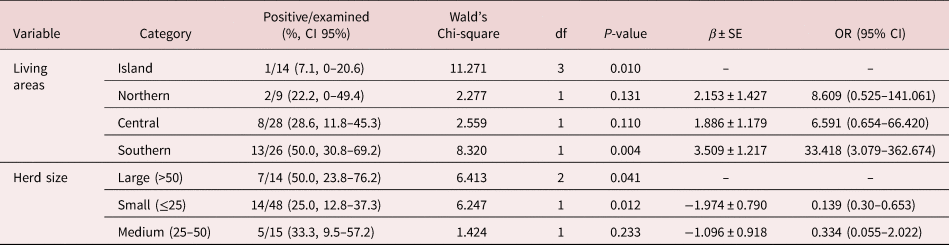
Results of logistic regression analysis for risk factors associated with patent infections of intestinal strongyles, Parascaris spp. and D. arnfieldi are summarized in tables 2–4. The results of the analysis at farm level of the presence of S. vulgaris are summarized in table 5. In the logistic regression analysis of intestinal strongyles, breed, co-pasture with horses, living area, herd size and number of treatments were significantly associated with the outcome variables (table 2).
Table 2. Distribution of frequencies and risk factors associated with intestinal strongyle infection in studied donkeys according to the multivariable generalized linear model.
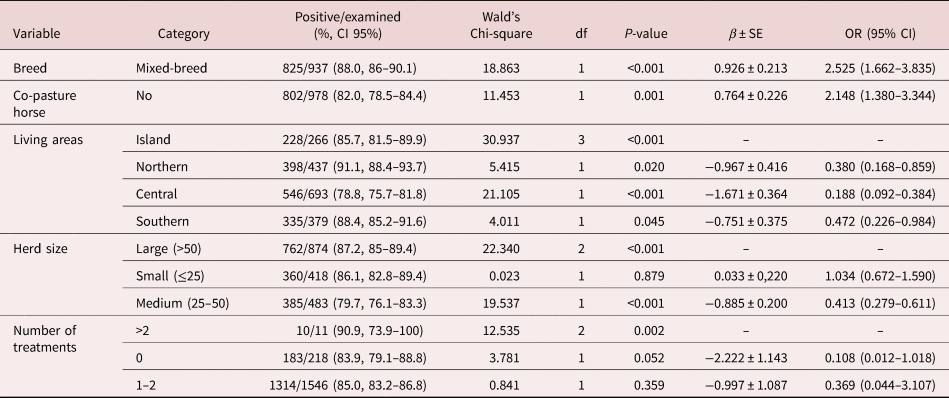
β ± SE, coefficient ± standard error; OR, odds ratio; CI, confidence interval.
Table 3. Distribution of frequencies and risk factors associated with Parascaris spp. infection in studied donkeys according to the multivariable generalized linear model.

β ± SE, coefficient ± standard error; OR, odds ratio; CI, confidence interval.
Table 4. Distribution of frequencies and risk factors associated with Dictyocaulus arnfieldi infection in studied donkeys according to the multivariable generalized linear model.
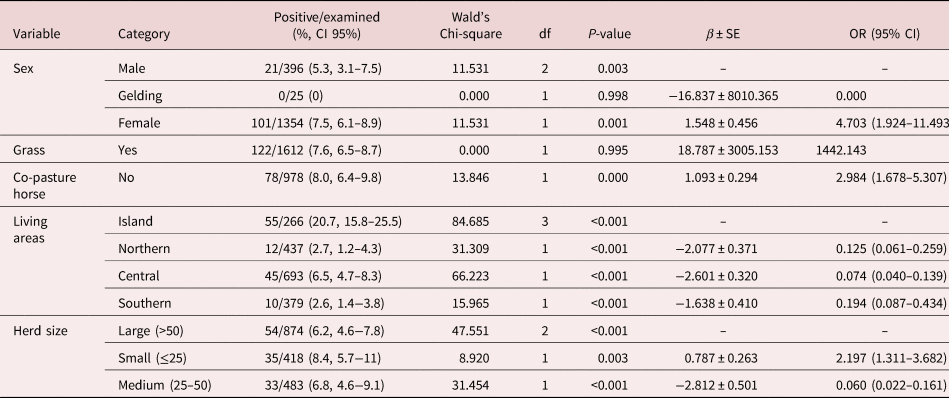
β ± SE, coefficient ± standard error; OR, odds ratio; CI, confidence interval.
Table 5. Distribution of frequencies and risk factors associated with Strongylus vulgaris infection in studied farms according to the multivariable generalized linear model.
P-value < 0.05. β ± SE, coefficient ± standard error; OR, odds ratio; CI, confidence interval.
In the logistic regression analysis of Parascaris spp., sex, age, living area and herd size were significantly associated with the outcome variables (table 3).
In the logistic regression analysis of D. arnfieldi, sex, grass, co-pasture with horses, living area and herd size were significantly associated with the outcome variables (table 4).
In the logistic regression analysis of S. vulgaris, living area and herd size were significantly associated with the outcome variable (table 5).
Discussion
The present study is the first European countrywide survey providing data on parasite dynamics in donkeys and provides valuable new information for the construction of meaningful parasite control programs.
The frequency of anthelmintic treatment reported in this study is quite different to what has been reported in horses in Italy (Veneziano et al., Reference Veneziano, Di Loria, Masucci, Di Palo, Brianti and Gokbulut2015), where deworming was carried out by almost all owners (94.0%) and the frequency was mainly two or three times/year (68.6%) and comparable with other equine surveys (Schneider et al., Reference Rode and Jorgensen2014; Nielsen et al., Reference Nielsen, Scare, Gravatte, Bellaw, Prado and Reinemeyer2018; Tzelos et al., Reference Trentini, Stancampiano, Usai, Micagni and Poglayen2019). Moreover, MLs (mainly IVM) were the most common drugs used to control endoparasites in Italian donkey farms, similarly to those used in horses (Veneziano et al., Reference Veneziano, Di Loria, Masucci, Di Palo, Brianti and Gokbulut2015; Becher et al., Reference Becher, van Doorn, Pfister, Kaplan, Reist and Nielsen2018; Nielsen et al., Reference Nielsen, Scare, Gravatte, Bellaw, Prado and Reinemeyer2018; Wilkes et al., Reference von Samson-Himmelstjerna, Traversa and Demeler2020). This supports the theory that deworming in donkeys is commonly performed with the same drugs and dosages used in horses (Gokbulut & McKellar, Reference Gokbulut and McKellar2018). In Italy, given that a sizeable proportion of donkeys are raised mainly for milk production for human consumption, the use of MLs (IVM and MOX) should be avoided in lactating animals given the lack of established withdrawal time for milk in equids (Gokbulut & McKellar, Reference Gokbulut and McKellar2018).
It is important to keep in mind that donkeys are not small horses with long ears, as they are characterized by a greater level of activity of some P450 isoenzymes than horses (Lizzaraga et al., Reference Lizzaraga, Sumano and Brumbaugh2004). Thus, for certain drugs, the recommended dose and posology for horses may not be appropriate for donkeys (Grosenbaugh et al., Reference Grosenbaugh, Reinemeyer and Figueiredo2011). Regarding anthelmintic drugs, some studies confirm the use of horse dosage in donkeys, despite the apparent differences in intestinal absorption and plasma disposition (Gokbulut & McKellar, Reference Gokbulut and McKellar2018). PYR pamoate in oral paste and granule formulations administered to donkeys at the horse dose rates, showed different bioavailability and persistence compared to horses, but were effective in controlling intestinal strongyle infections (Gokbulut et al., Reference Gokbulut, Aksit, Smaldone, Mariani and Veneziano2014). In donkeys, mebendazole was reported to be highly effective against small strongyles both at the horse dose rate and at double the horse dosage, and egg reappearance period was five weeks for both dosages (Gokbulut et al., Reference Gokbulut, Aksit, Santoro, Roncoroni, Mariani, Buono, Rufrano, Fagiolo and Veneziano2016). In donkeys, a recent trial showed that in well-managed farms, anthelmintic drugs administered at the horse dose rate were generally effective in the control of small strongyle infections (Buono et al., Reference Buono, Roncoroni, Pacifico, Piantedosi, Neola, Barile, Fagiolo, Várady and Veneziano2018). Based on the results of these studies, the horse dosage for some anthelmintics may also be effective for the donkey dosage. In the present survey, owners and veterinarians generally treated donkeys like horses.
Intestinal strongyle infections are common in grazing horses and donkeys; however, in this survey, access to pasture was not associated with a higher prevalence of positive strongyle FECs. A similar finding was made in a previously published equine study (von Samson-Himmelstjerna et al., Reference Veneziano, Veronesi and Buono2009). Donkeys raised in box stalls with an annexed paddock showed high intestinal strongyle prevalence, and it is possible that parasites could be transmitted in stalls in cases of suboptimal hygiene (Nielsen et al., Reference Nielsen, Vidyashankar, Andresen, De Lisi, Pilegaard and Kaplan2010b). Moreover, it should keep in mind that encysted and arrested larvae will gradually develop into adult parasites, causing positive FECs over time, and several anthelmintic treatments could be needed to achieve a negative FEC.
In the examined farms, donkeys generally grazed with a low number of ruminants, and the ratio between these two species was very low. This would explain why a ‘dilution effect’ was not observed and why there were no significant differences in strongyle egg excretion between donkeys that co-grazed and those that did not co-graze with ruminants.
Donkeys raised in farms in northern Italy had a significantly higher prevalence of strongyle infection. This is probably due to the climatic difference between northern and central-southern Italy. Pasture contamination with small strongyles is widely influenced by climatic conditions (Leathwick et al., Reference Leathwick, Donecker and Nielsen2015); in northern Italy, rainfalls are more abundant than central-southern Italy and this would allow a greater migration of the L3 on the pastures.
Small (≤25 animals) and large farms (≥50 animals) were more associated with strongyle infection than medium-sized farms (25–50 donkeys). Similar findings have been reported in horses (Kornaś et al., Reference Kornaś, Cabaret, Skalska and Nowosad2010). In small farms, donkeys are usually housed with horses and are often neglected and just sheltered into the farms without being involved in management and anthelmintic control practices, whereas in larger farms a high stocking density could be correlated to higher infection pressure.
The distribution of strongyle egg count levels reported herein are different from those reported in managed horses, where an overwhelming majority (50–75%) is in the low contaminator category (Relf et al., Reference Proudman and Edwards2013; Lester et al., Reference Lester, Morgan, Hodgkinson and Matthews2018). Consequently, the 80:20 distribution rule that is often cited for horses (Becher et al., Reference Becher, Mahling, Nielsen and Pfister2010; Nielsen et al., Reference Nielsen, Scare, Gravatte, Bellaw, Prado and Reinemeyer2018), according to which 20% of animals shed about 80% of intestinal strongyle eggs, does not appear directly applicable for this donkey population. Here, about 40% of adult donkeys were shedding approximately 80% of the eggs, suggesting an 80:40 distribution (fig. 3). In this study, 770 donkeys (43.4%) had <300 EPG intestinal strongyles, and, thus, these animals may not require deworming.

Fig. 3. This graph presents the overdispersion in the intestinal strongyles faecal egg count (FEC) in donkeys (80:40 rule). In the study donkeys (1775), 1005 animals (56.6%) were above the red threshold line, with FEC > 300 eggs per gram (EPG), while 770 (43.4%) were below the red threshold line, with FEC < 300 EPG.
Faecal cultures documented a wide biodiversity with a high prevalence of large strongyles, most notably S. vulgaris. Given the distinct pathogenicity of this parasite (Pihl et al., Reference Otranto and Dantas-Torres2018), more attention should be given to controlling it in donkey operations. It was reported that S. vulgaris caused obstruction of the cranial mesenteric artery, which led to death of the animal in an eight-year-old donkey (Borji et al., Reference Borji, Moosavi and Ahmadi2014). Moreover, in a two-year-old intact male donkey, aberrant migrations of S. vulgaris through the spinal cord resulted in progressive paraparesis and tetraplegia (Mayhew et al., Reference Matthews and Lekeux1984). The farm-level analysis showed a significant association between S. vulgaris and location, suggesting that donkeys raised on farms located on the island of Sicily are less exposed to S. vulgaris infection. The reason for this difference is not clear, but climatic influence and management practices may play a role.
The low anthelmintic treatment intensity reported in this study could be a risk factor for S. vulgaris infection in donkeys as also demonstrated in horses (Nielsen et al., Reference Nielsen, Fritzen and Duncan2012; Hedberg-Alm et al., Reference Hedberg-Alm, Pennell, Riihimäki, Osterman-Lind, Nielsen and Tydén2020). Considering the high prevalence of S. vulgaris in the donkey population and that many donkeys co-graze with horses, it is necessary to assess the presence of large strongyles in donkey species to avoid the risk of infection in horses. Thus, if coprocultures show the presence of S. vulgaris, it is important to also treat the low contaminators (FEC <300 EPG) to limit its spread on the pasture. It is also important to highlight that IVM treatment may not be completely effective against S. vulgaris migrating fifth-stage larvae (Nielsen et al., Reference Nielsen, Vidyashankar, Olsen, Monrad and Thambsborg2015). Although horses demonstrate different degrees of immunity to Parascaris spp. in relation to age and exposure with patent infections (Clayton & Duncan, Reference Clayton and Duncan1979), adult donkeys can harbour ascarids without clinical signs, and could be playing an important role in pasture contamination (Matthews & Burden, Reference Matthews and Lekeux2013). The reason for female donkeys having a lower ascarid prevalence than males is unclear, but could be due to differences in management strategies. A large proportion of females were kept for milk production purposes, whereas males were used for a variety of purposes, including meat production. Consequently, males and females could be managed substantially differently, which could affect the risk of acquiring patent ascarid infection.
Although ascarid eggs were found in faeces of all age groups, younger donkeys (≤1 year) had a significantly higher prevalence than other age groups. This contrasts findings among working donkeys in Ethiopia, where a high prevalence (>50%) regardless of the animal age was reported (Getachew et al., Reference Getachew, Innocent, Trawford, Feseha, Reid and Love2008). This discrepancy could be explained by the inability of the animals to develop immunity against ascarids due to poor farm management and poor nutrition for working equids in developing countries (Getachew et al., Reference Getachew, Trawford, Feseha and Reid2010). Donkeys on farms located in southern Italy had significantly higher Parascaris spp. prevalence, and this can be explained considering that in these farms donkeys were kept mainly for milk production and, therefore, characterized by a greater presence of animals aged less than one year.
Donkeys and mules are natural hosts for D. arnfieldi, whereas horses are primarily at risk of getting infected while co-grazing with these animals. Although the prevalence was relatively low in this study, it could be underestimated considering that the first-stage larvae (L1) recovery of D. arnfieldi by Baermann technique is strongly influenced by the method of storage of faecal samples. Considering that faecal samples were stored in portable refrigerators, temperature fluctuations during the first 48 h could adversely affect the parasitological examination (Rode & Jorgensen, Reference Relf, Morgan, Hodgkinson and Matthews1989). Female donkeys were at higher risk of infection than male and gelding, probably due to the fact that they spent more time grazing. Donkeys that had access to pasture were at major risk of infection by D. arnfieldi, suggesting that animals acquire infections when grazing on a contaminated pasture (Matthews, Reference Matthews and Burden2002). Donkeys co-grazing with horses had a lower prevalence of D. arnfieldi, which is probably due to the dilution principle, considering that horses rarely harbour adult stages and considering that horses are not permissive hosts of the full life cycle, they generally do not excrete larvae in the faeces (Matthews & Burden, Reference Matthews and Burden2013). The highest prevalence was reported in animals aged less than one year and in animals with BCS<3. This makes sense as young age and poor BCS represent potential risk factors, probably related to impaired immunity to infections (Tihitna et al., Reference Tihitna, Basaznew, Mersha and Achenef2007). It is advisable to perform diagnosis for lungworm infection in all donkeys co-grazing with horses, and the animals should be treated using MLs to reduce pasture contamination by L1 of D. arnfieldi (Matthews, Reference Matthews and Burden2002). In our study, IVM was the compound mostly used for deworming and this could explain the low prevalence of lungworm infections. Moreover, the frequency of anthelmintic treatments was associated with a lower infection rate, suggesting that the routine deworming scheme in the donkey farms could be correlated to a reduction of lungworm parasitic load.
The prevalence of Anoplocephala spp. was low (1.0%) but in agreement with data reported in donkeys in few studies on the presence of tapeworms in Europe (Matthews & Burden, Reference Matthews and Lekeux2013). In horses, Anoplocephala perfoliata was detected with higher prevalence (Tzelos et al., Reference Trentini, Stancampiano, Usai, Micagni and Poglayen2019; Hedberg-Alm et al., Reference Hedberg-Alm, Pennell, Riihimäki, Osterman-Lind, Nielsen and Tydén2020). This low prevalence could be correlated with the irregular excretion of tapeworm eggs in the faeces (Gasser et al., Reference Gasser, Williamson and Beveridge2005). Furthermore, considering that the Proudman test can result in false negatives if the parasitic load is less than 20 worms (Proudman & Edwards, Reference Pihl, Nielsen, Olsen, Leifsson and Jacobsen1992), it is possible that tapeworm infection was not diagnosed in those animals with a low parasitic load.
In Europe, F. hepatica is widespread in ruminants, but with a clustered spatial distribution, and regional variation in epidemiology and prevalence (4% in southern Italy) (Beesley et al., Reference Beesley, Caminade and Charlier2017). In Italy, the low prevalence of this liver fluke could justify its absence in the studied donkey population.
In conclusion, this study represents the first large-scale epidemiological survey in Europe on the prevalence of helminth infections in donkeys, adding useful data to the scarce literature available about the parasite dynamics in this neglected animal. Our findings revealed the presence of several helminth species, representing the most important pathogenic parasites of equids and donkeys worldwide. Considerations for parasite control in donkeys could be different to those suggested in horses, because large strongyles in donkeys are more common than in horses. Furthermore, in donkeys the 80:20 strongyle egg shedding distribution rule is not applicable, because about 40% of donkeys were shedding around 80% of the eggs. Furthermore, this study highlighted the prominent occurrence of S. vulgaris in managed donkeys and a farm prevalence exceeding that of D. arnfieldi. Thus, parasite control programs in donkeys require specific consideration of strongyle egg shedding patterns as well as occurrence of individual parasite species with pathogenic potential for both the donkeys and possible co-grazing horses.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X20001017.
Acknowledgements
This work would not be possible without the willingness of the donkey owners who agreed to participate in the survey and Il Rifugio degli Asinelli (The Donkey Sanctuary branch) that supported the research.
Author contributions
VV, BF, PL and RC contributed conception and design of the study. BF, FV, PL, NE, SG, NB, MU and ZSA collected the samples, performed the laboratory activities and organized the database. BF, PD and VV wrote the manuscript. VF performed statistical analysis. MKN critically oversaw substantial revisions of the manuscript. All of the authors revised the manuscript and approved the submitted version.
Financial support
This study was partially funded by grants from the Ministry of Health of the Italian Republic (IZSME 14/11 RC and IZSLT 11/15 RC, IZSLT 9/18 RC).
Conflicts of interest
None.










